Terashima and co-workers report efficient synthetic systems of single-chain crosslinked polymers.
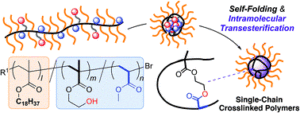
Crosslinked polymers have emerged as a class of unique materials which find use in a diverse range of applications such as drug delivery, dispersants and coating industries. Typically, those materials are made through a combination of controlled polymerization and crosslinked methods. In this work, Terashima and co-workers prepared a range of single-chain crosslinked polymers with controlled crystallization. This was achieved by the intramolecular transesterification of random copolymers compromising of octadecyl methacrylate, 2-hydroxyethyl methacrylate, and methyl acrylate. Those copolymers were self-folded in organic media (octane was used as the solvent) through the association of the hydroxyl groups to form reverse micelles. Upon synthesis, the micelles were intramolecularly crosslinked by an efficient transesterification of the methyl acrylate units with the hydroxyl groups to produce polymer nanoparticles with pending octadecyl groups. The materials synthesized were thoroughly characterized by a number of techniques including nuclear magnetic resonance, gel permeation chromatography, small angle X-ray scattering and dynamic light scattering. The developed system allowed for the efficient control of the molecular weight of the crosslinked polymers owing to the precise synthesis of the precursors prepared by living radical polymerization. Importantly, the degree of crosslinking was found to control the crystallinity of the products. Last but not least, a relatively high concentration could be used (up to 50 mg ml-1). As the authors allude to in their conclusion, their work has paved the way to the production of well-defined polymeric nanoparticles that can be employed for surface coating, painting, optical plastics and cosmetics.
Tips/comments directly from the authors:
1) Intramolecular crosslinking of folded polymers in organic media via transesterification affords the precision and high-throughput synthesis of single-chain crosslinked polymer nanoparticles.
2) The molecular weight of the crosslinked polymers can be controlled as desired at the stage of the synthesis of the precursor polymers by controlled radical polymerization.
3) Transesterification between hydroxyl groups and methyl acrylate units efficiently proceeds within the cores of folded micelles to fix the folded structures in a specific solvent.
4) SEC-MALLS analysis is essential to characterize single-chain crosslinked polymers. Because of the compact structures, the apparent molecular weight of the crosslinked polymers by the general RI detector with PMMA standard calibration turns smaller than that of the non-crosslinked precursor polymers. If the absolute weight-average molecular weight of the crosslinked polymers by the MALLS detector is also close to that of the precursor polymers, you can conclude that the products consist of single chain-crosslinked polymers.
5) Crystallinity of the bulk polymers is controlled by tuning the degree of intramolecular crosslinking. This is an interesting approach to control the thermal and physical properties of solid polymer materials.
Citation to the paper: Single-chain crosslinked polymers via the transesterification of folded polymers: from efficient synthesis to crystallinity control, Polym. Chem., 2020, 11, 5181-5190, doi.org/10.1039/D0PY00758G
Link to the paper: https://pubs.rsc.org/en/content/articlepdf/2020/py/d0py00758g
About the web writer:
Dr. Athina Anastasaki is an Editorial Board Member and a Web Writer for Polymer Chemistry. Since January 2019, she joined the Materials Department of ETH Zurich as an Assistant Professor to establish her independent research group.