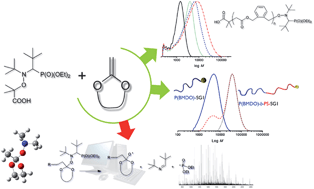
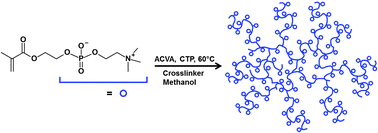
In this paper, Huang, Guo and co-workers developed a novel micellar cross-linking copolymerization method to prepare highly stretchable and resilient hydrogels. The polymerization was based on free-radical copolymerization of water soluble acrylamide and a polymerizable macromolecular surfactant (i.e., amphiphilic polyurethane macromonomer) which can self-assemble into micelles acting as multifunctional cross-linkers. The mechanical properties, such as breaking elongation ratio, modulus and fracture toughness can be easily adjusted by varying the concentration of the polymerizable macromolecular surfactants. In addition, the mechanical energy storage efficiency (also known as resilience) was more than 96% at a strain up to 400%. These findings established a strategy for the preparation of hydrogels that combine high extendibility with excellent resilience and may greatly benefit the further use of hydrogels in tissue engineering and other soft materials research fields.
Highly stretchable and resilient hydrogels from the copolymerization of acrylamide and a polymerizable macromolecular surfactant by Mei Tan, Tingting Zhao, He Huang and Mingyu Guo Polym. Chem. 2013, 4, 5570-5576.
Julien Nicolas is a guest web-writer and advisory board member for Polymer Chemistry. He currently works at Univ. Paris-Sud (FR) as a CNRS researcher.












![Graphical abstract: Direct heteroarylation of β-protected dithienosilole and dithienogermole monomers with thieno[3,4-c]pyrrole-4,6-dione and furo[3,4-c]pyrrole-4,6-dione](http://pubs.rsc.org/services/images/RSCpubs.ePlatform.Service.FreeContent.ImageService.svc/ImageService/image/GA?id=C3PY21138J)