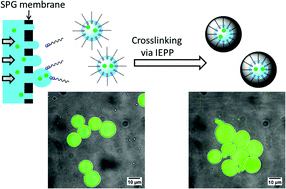
Ishizuka et al. report the synthesis of polymeric microcapsules with a hydrophilic core using SPG membrane emulsification.
Polymeric nano/micro capsules can be used in many applications ranging from biomedicine to cosmetics, food industry and water treatment. However, the current approaches to prepare hollow particles possess a number of drawbacks including tedious and multistep synthesis and low encapsulation efficiency. In order to address these limitations, Zetterlund, Stenzel and co-workers have elegantly combined inverse emulsion periphery reversible addition-fragmentation chain transfer polymerization (RAFT) polymerization (IEPP) with shirasu porous glass membrane (SPG) emulsification to synthesize hollow particles with a hydrophilic core. An amphiphilic macroRAFT agent, namely poly(di (ethylene glycol)methyl ether methacrylate)17-block-poly (methyl methacrylate), was employed to prepare inverse emulsions with a relatively narrow size distribution. Adjusting the SPG membrane pore size from 0.2 to 3 μm allowed access to good control over the droplet size and subsequently the size of the final capsules. This is a significant improvement over conventional emulsions based techniques (e.g. (mini)emulsion or suspension systems) which typically generate either smaller or larger particles. Importantly, the synthesized emulsions were stable at room temperature for over a month maintaining their size and size distribution post polymerization. Finally, the authors demonstrated the successful encapsulation of a water soluble fluorescent dye without any significant effect on the droplet/microcapsule diameter. Therefore, this process showed great potential and can be applied to a wide range of droplets, enabling the encapsulation of various molecules in nano/micro capsules.
Tips/comments directly from the authors:
1. It is important to use a “hydrophobic membrane” to prepare inverse emulsions. Membranes are reusable after cleaning. Typically after each experiment, the membrane is first washed with THF to remove polymer, then with water to remove salt/water soluble guest molecules, subsequently with THF and finally with toluene at least three times to make sure the membrane is wetted with toluene.
2. In order to prepare monodisperse droplets, the continuous phase has to be stirred gently (in this study 160-170 rpm) during emulsification to create a flow which induces the detachment of droplets from the membrane surface and to prevent creaming (generated droplets would settle down without stirring). The stirring speed may differ depending on the specific emulsification condition.
3. During the crosslinking polymerization, a higher stirring rate (500-600 rpm) is typically required to prevent aggregation of particles. The optimum stirring rate may differ depending on the size of the reaction vessel and/or volume of the reaction mixture.
Read this exciting research for free until 25/02/2017 through a registered RSC account:
Synthesis of microcapsules using inverse emulsion periphery RAFT polymerization via SPG membrane emulsification
Polym. Chem., 2016, 7, 7047-7051, DOI: 10.1039/c6py01584k
—————-
Dr. Athina Anastasaki is a web writer for Polymer Chemistry. She is currently an Elings fellow working alongside Professor Craig Hawker at the University of California, Santa Barbara (UCSB). Please visit this website for more information.