To keep up-to-date with all the latest research, sign up for the journal’s e-alerts or RSS feeds or follow Polymer Chemistryon Twitter or Facebook.
Author Archive
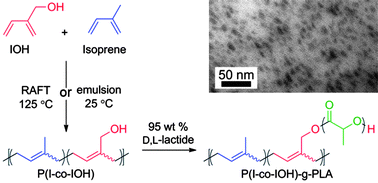
Paper of the Week: Copolymerization of isoprene and hydroxyl containing monomers by controlled radical and emulsion methods
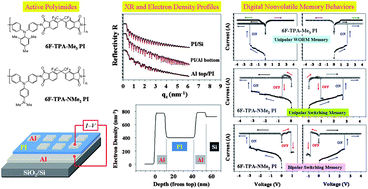
Paper of the Week: Programmable digital nonvolatile memory
To keep up-to-date with all the latest research, sign up for the journal’s e-alerts or RSS feeds or follow Polymer Chemistryon Twitter or Facebook.
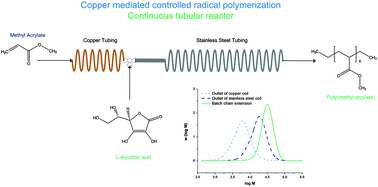
Paper of the Week: Copper mediated controlled radical polymerization in a continuous tubular reactor
To keep up-to-date with all the latest research, sign up for the journal’s e-alerts or RSS feeds or follow Polymer Chemistryon Twitter or Facebook.
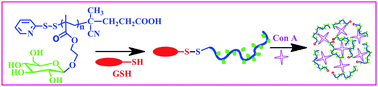
Paper of the Week: Glycopolymer–peptide bioconjugates with antioxidant activity
To keep up-to-date with all the latest research, sign up for the journal’s e-alerts or RSS feeds or follow Polymer Chemistryon Twitter or Facebook
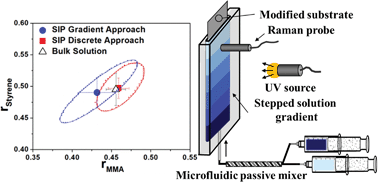
Paper of the Week: Measurement platform for monomer reactivity ratios
To keep up-to-date with all the latest research, sign up for the journal’s e-alerts or RSS feeds or follow Polymer Chemistryon Twitter or Facebook
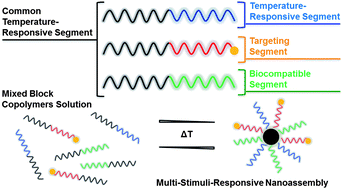
Paper of the Week: Multifunctional nano-assemblies
To keep up-to-date with all the latest research, sign up for the journal’s e-alerts or RSS feeds or follow Polymer Chemistry on Twitter or Facebook
Paper of the Week: Redox-sensitive shell cross-linked PEG–polypeptide hybrid micelles for controlled drug release
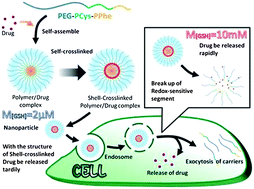
To keep up-to-date with all the latest research, sign up for the journal’s e-alerts or RSS feeds or follow Polymer Chemistryon Twitter or Facebook
Paper of the Week: Jellyfish-shaped amphiphilic block-graft copolymers through ATRP, ROP and click chemistry
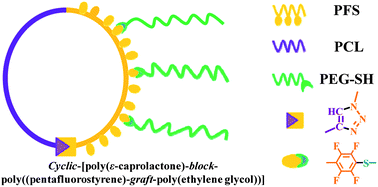
Preparation of jellyfish-shaped amphiphilic block-graft copolymers consisting of a poly(3-caprolactone)-block-poly(pentafluorostyrene) ring and poly(ethylene glycol) lateral brushes by Tao Cai, Wen Jing Yang, Koon-Gee Neoh and En-Tang Kang Polym. Chem. 2012, 3, 1061-1068.
To keep up-to-date with all the latest research, sign up for the journal’s e-alerts or RSS feeds or follow Polymer Chemistryon Twitter or Facebook.
Paper of the Week: High molecular weight acrylonitrile–butadiene architectures designed by a RAFT/click chemistry toolbox
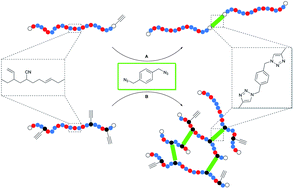
High molecular weight acrylonitrile–butadiene architectures via a combination of RAFT polymerization and orthogonal copper mediated azide–alkyne cycloaddition by Christoph J. Dürr , Sebastian G. J. Emmerling , Paul Lederhose , Andreas Kaiser , Sven Brandau , Michael Klimpel and Christopher Barner-Kowollik Polym. Chem. 2012, 3, 1048-1060.
To keep up-to-date with all the latest research, sign up for the journal’s e-alerts or RSS feeds or follow Polymer Chemistryon Twitter or Facebook.
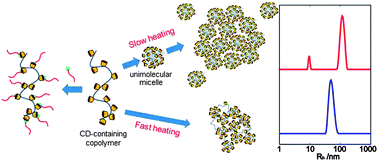
Polymer Chemistry Paper of the Week: A new story of cyclodextrin as a bulky pendent group causing uncommon behaviour to random copolymers in solution
A new story of cyclodextrin as a bulky pendent group causing uncommon behaviour to random copolymers in solution by Fuji Sakai, Guosong Chen and Ming Jiang Polym. Chem. 2012, 3, 954-961.
To keep up-to-date with all the latest research, sign up for the journal’s e-alerts or RSS feeds or follow Polymer Chemistryon Twitter or Facebook.