Author Archive
12 Mar 2013
Block copolymers are interesting materials that can self-assemble and segregate on nanometer length scales. This makes them ideal materials for numerous applications ranging from conventional technologies to emerging nanotechnologies. The synthesis of block copolymers is generally achieved via “living” ionic polymerization techniques or reversible-deactivation radical polymerization methods. However, the synthesis of well-defined block copolymers is, in general, a relatively low throughput, demanding, expensive and time-consuming process.
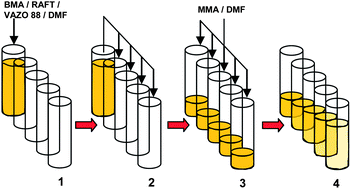
In this context, Chiefari and co-workers reported a convenient synthetic method for the systematic preparation of quasi-diblock copolymer libraries utilizing a sequential reversible addition–fragmentation chain transfer (RAFT) polymerization strategy. This approach used a commercially available parallel synthesizer, which allowed the unattended and fully automated synthesis of these libraries in a short period of time. The materials obtained in this investigation have shown properties very similar to those expected in “pure” diblock copolymers as determined by differential scanning calorimetry. The described method can be a useful and less expensive alternative for the rapid preparation and screening of block copolymer libraries.
Quasi-block copolymer libraries on demand via sequential RAFT polymerization in an automated parallel synthesizer by Carlos Guerrero-Sanchez, Lisa O’Brien, Colin Brackley, Daniel J. Keddie, Simon Saubern and John Chiefari, Polym. Chem., 2013, 4, 1857-1862.
Julien Nicolas is a guest web-writer for Polymer Chemistry. He currently works at Univ. Paris-Sud (FR) as a CNRS researcher.
Comments Off on Paper of the week: Quasi-block copolymer libraries from an automated parallel synthesizer
06 Mar 2013
The stabilization of liquid–liquid interfaces through the adsorption of surfactants plays an important role in many industrial processes in which emulsions are produced or used. The main aim in the development of new surfactants is to provide prolonged shelf-life by preventing the coalescence of emulsion droplets. While the stability of an emulsion against coalescence is often the main aim in the formulation of emulsion systems, many applications of emulsions do not only require high stability against coalescence, but also the breaking or phase inversion of the emulsion at some point. At first, these requirements seem contradictory: the emulsion needs to be stable on the shelf, yet becomes unstable at some moment under the influence of a given stimulus.
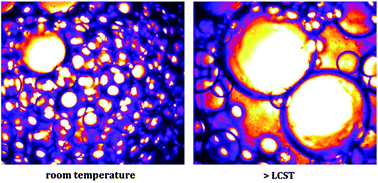
In this context, Sprakel and co-workers approached these contradictory constraints through the synthesis of well-defined thermoresponsive surfactants based on di(ethylene glycol)methacrylate and poly(ethylene glycol)methacrylate using Atom Transfer Radical Polymerization. The surfactants showed a Lower Critical Solution Temperature (LCST) of approximately 34°C, independent of molecular weight, which is ascertained by both Differential Scanning Calorimetry as well as Dynamic Light Scattering. Below the LCST, the surfactants stabilized the emulsions for at least four months. Above this temperature the hydrophilic block collapsed and coalescence between the emulsion droplets occurred; this led to demixing of the sample within several minutes. The authors revealed the mechanism for the temperature-triggered coalescence by measurements of the temperature-dependent interfacial tension and by studying the interfacial morphology of surfactant-covered emulsion droplets.
Well-defined temperature-sensitive surfactants for controlled emulsion coalescence by Huanhuan Feng, Nadine A. L. Verstappen, Alexander J. C. Kuehne and Joris Sprakel, Polym. Chem., 2013, 4, 1842-1847.
Julien Nicolas is a guest web-writer for Polymer Chemistry. He currently works at Univ. Paris-Sud (FR) as a CNRS researcher.
Comments Off on Paper of the week: Well-defined temperature-sensitive surfactants for controlled emulsion coalescence
28 Feb 2013
Due to the properties arising from the synergism between the polyolefins and composites bearing polar functionalities, the unique behavior of these materials in bulk and in solution has led to emerging research in this area. Broad scientific effort, both fundamental and applied, has been devoted to obtain well-defined statistical, block and graft copolymers by covalently bonding polyolefins to amphiphilic polymers. It is well known that sequence distributions are major contributors in the adjustment of material properties in copolymers.
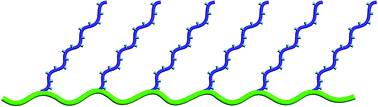
In this context, Markova et al. intended to use the synthetic advantages of acyclic diene metathesis (ADMET) polymerization (which offers a synthetic route to strictly linear polyethylenes functionalized at precise intervals) for the formation of new membrane materials for low temperature fuel cells. Well-defined, precise poly(vinylbenzyl phosphonic acid) (PVBPA)-containing-polyolefins were obtained for the first time by a combination of ADMET and atom transfer radical polymerization (ATRP), yielding a set of well-defined graft copolymers composed of polyethylene (PE) “backbone” and PDEVBP brushes, precisely placed on every 21st carbon. Quantitative deprotection of the phosphonates led to the corresponding polymer bonded phosphonic acids. The PA-containing electrolytes exhibited sufficient thermal properties with a high mobility for proton conduction. Moreover, the enhancement of the proton conductivity properties compared to the existing phosphonic acid containing block copolymer structures, the improved properties in the high temperature operating regime and the conductivity range make them interesting membrane materials for future studies.
Synthesis of proton conducting phosphonic acidfunctionalized polyolefins by the combination of ATRP and ADMET by Dilyana Markova, Kathleen L. Opper, Manfred Wagner, Markus Klapper, Kenneth B. Wagener and Klaus Mullen, Polym. Chem., 2013, 4, 1351-1363.
Julien Nicolas is a guest web-writer for Polymer Chemistry. He currently works at Univ. Paris-Sud (FR) as a CNRS researcher.
Comments Off on Paper of the week: Proton conducting phosphonic acid-functionalized polyolefins
18 Feb 2013
pH-sensitive anionic dyes have been widely used in scientific research and industrial applications. The most common pH test approach generally utilizes papers as the substrate which absorbed pH-sensitive dyes. However, pH test papers often suffer from the leaching of dyes into solution, and the contamination of the samples which can result in anomalous readings. In the past years, plastic pH-sensitive strips have been prepared by immobilizing small molecular pH-sensitive dyes into the polymer matrix via the adsorption or entrapment approach. However, the physical entrapment may lead to a gradual loss of dyes from the substrates, which therefore limits the sensor stability and long-term practical applications. Therefore, various polymers with covalently bonded pH-sensitive moieties have been recently developed for the pH test.
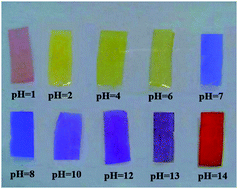
In their study, Yan and co-workers reported a facile and effective strategy for the preparation of plastic pH indicator strips and the characterization of their pH sensitivity. They focused their attention on poly(ionic liquids) (PILs) due to their excellent ion exchange capability, enabling the preparation of PILs with a variety of counteranions by polymerization of only one IL monomer and followed by anion-exchange reactions. The pH-sensitive strips were prepared via the cross-linking of imidazolium type IL monomers with acrylonitrile and followed by anion-exchange with sulfonated anionic dyes which bear their negative charge in a wide pH range. The resultant pH indicator strips exhibited enhanced pH-responsive colour changes and robust pH-response reversibility in both aqueous and organic solutions.
Plastic reusable pH indicator strips: preparation via anion-exchange of poly(ionic liquids) with anionic dyes by Jiangna Guo, Lihua Qiu, Zhijun Deng and Feng Yan, Polym. Chem., 2013, 4, 1309-1312.
Julien Nicolas is a guest web-writer for Polymer Chemistry. He currently works at Univ. Paris-Sud (FR) as a CNRS researcher.
Comments Off on Paper of the week: Plastic reusable pH indicator strips
12 Feb 2013
Supramolecular polymers, defined as polymeric arrays of many repeating units held together by reversible and weak non-covalent interactions, are considered as the result of the combination of supramolecular chemistry and polymer science. Non-covalent interactions, such as multiple hydrogen bonding, host–guest interactions and metal-coordination, have been introduced to fabricate supramolecular polymers, and these reversible and highly directional secondary interactions endow supramolecular polymers with novel topological structures and unique functions. Charge transfer complexes, prepared by the association between an electron acceptor and an electron donor, have been proved to be important and attractive building blocks for the construction of multiple supramolecular aggregates.
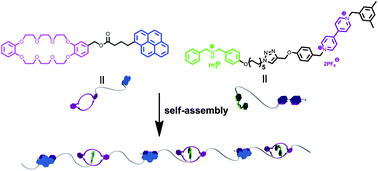
In this view, Huang and co-workers reported on the design and the synthesis of a novel supramolecular polymer constructed from two heteroditopic monomers driven by the combination of crown ether-based and charge-transfer molecular recognition. High molecular weight supramolecular polymers were formed by complexation between crown ethers and secondary ammonium salts, and paraquat derivatives and pyrene derivatives, respectively. This kind of supramolecular polymer exhibited the ability to construct nanofibers via electrospinning technology.
A supramolecular polymer formed by the combination of crown ether-based and charge-transfer molecular recognition by Shengyi Dong, Lingyan Gao, Jianzhuang Chen, Guocan Yu, Bo Zheng and Feihe Huang, Polym. Chem., 2013, 4, 882-886.
Julien Nicolas is a guest web-writer for Polymer Chemistry. He currently works at Univ. Paris-Sud (FR) as a CNRS researcher.
Comments Off on Paper of the week: A novel supramolecular polymer via host–guest and charge-transfer interactions
05 Feb 2013
A wide range of pharmaceuticals that are not completely metabolized in humans or animals are discharged into aquatic environments through a variety of sources including homes, hospitals, pharmaceutical manufactures, and animal feeding operations. The potential hazard of pharmaceuticals in waters depends on their persistence and the biological activity of their degradation by-products; some of them have been demonstrated to be harmful not only to ecosystems but also to human health. Consequently, there is a need for their detection at trace levels and efficient removal from waters. Both applications require sorbents with enhanced binding properties of the targeted pharmaceuticals.
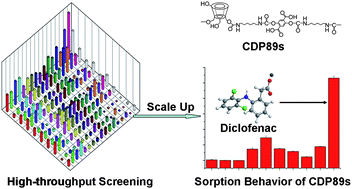
In oder to develop materials with recognition properties, Shahgaldian used cyclodextrins (CDs) for their known ability to include hydrophobic drugs into their cavity. Nevertheless, as the range of molecules known to form inclusion complexes with CDs is fairly broad, one can expect that the produced polymers will exhibit a lack of selectivity. With this in mind, 51 water-insoluble cyclodextrin-based polyurethanes were synthesized using a high-throughput approach. The selective molecular recognition properties of the produced polyurethanes were investigated by measuring their capability to bind ten selected compounds from aqueous solutions. Interestingly, the results indicated that the influence of different CDs on the selective molecular recognition properties was fairly limited. It was demonstrated that the selective molecular recognition properties can be suitably designed and optimized by tuning their compositions and successfully applied for selective binding of targets under purely aqueous conditions.
Design and high-throughput synthesis of cyclodextrin-based polyurethanes with enhanced molecular recognition properties by Pu Xiao , Philippe F.-X. Corvini , Yves Dudal and Patrick Shahgaldian, Polym. Chem., 2013, 4, 942-946.
Julien Nicolas is a guest web-writer for Polymer Chemistry. He currently works at Univ. Paris-Sud (FR) as a CNRS researcher.
Comments Off on Paper of the week: High-throughput synthesis of cyclodextrin-based polyurethanes
29 Jan 2013
Magnetite and maghemite are of particular interest is their use in the biomedical area, especially for applications related to targeted drug delivery, immunoassays, biomacromolecule purification, clinical diagnosis and therapy, such as magnetic resonance imaging (MRI), and magnetic fluid hyperthermia (MFH). For biomedical applications, the surface of the magnetite nanoparticles needs to be suitably engineered to acquire improved colloidal stability in physiological media (avoiding agglomeration), biocompatibility, drug encapsulation ability, and specific target ability to ensure desirable interactions with cells or tissues.
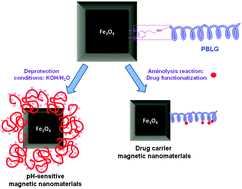
Having that in mind, the study of Marcelo and co-workers presents the synthesis of hybrid magnetic–polypeptide nanoparticles using a novel approach; that is the combination of dopamine employed as a bioinspired adhesive for a hydrophobic magnetite surface, acting at the same time as an initiator of the ring opening polymerization of g-benzyl-L-glutamate N-carboxyanhydride. Further deprotection allowed the authors to achieve poly(glutamic acid) chains, leading to pH sensitive hybrid materials. In addition, to explore the potential application of this system for drug carriers, the incorporation of a drug model compound, procaine, was also performed. Therefore, the resulting novel hybrid magnetite–polypeptide materials could be considered as good candidates for biomedical applications, such as contrast agents for magnetic resonance imaging, hyperthermia and drug delivery.
Hybrid materials achieved by polypeptide grafted magnetite nanoparticles through a dopamine biomimetic surface anchored initiator by G. Marcelo, A. Muñoz-Bonilla, J. Rodríguez-Hernández and M. Fernández-García, Polym. Chem., 2013, 4, 558-567.
Julien Nicolas is a guest web-writer for Polymer Chemistry. He currently works at Univ. Paris-Sud (FR) as a CNRS researcher.
Comments Off on Paper of the week: polypeptide-grafted magnetite nanoparticles
21 Jan 2013
Synthetic polymers have experienced an exponential growth over the last sixty years, in part due to their combined low density and exceptional mechanical properties. Along with chain architecture and microdomain morphology, polymer crystallinity is one of the determining parameters which control these properties. Recently, there has been a flurry of activity regarding the possibility of altering mechanical properties by changing chain architecture or microdomain morphology upon the action of a chemical stimulus, a property which can be exploited for the fabrication of mechanically adaptive objects as well as sensors.
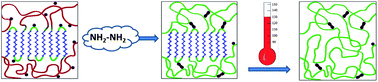
In this context, Claverie and co-workers reported a functional polymer with chemically switchable crystallinity. Linear polyethylenes containing pendant diacetone acrylamide groups were prepared using Pd phosphine sulfonate catalysts. These polymers are easily cross-linked upon reaction with hydrazine, and the cross-links can be cleaved by ozonolysis to regenerate the original polymer. The cross-linked polymer, once heated above the melting point, becomes permanently amorphous, as the crosslinks prevent the chains from packing. Crystallinity can only be recovered with the cleavage of the cross-links. Thus, the polymeric material exhibits two states (crystalline and amorphous) which are triggered upon action of simple chemicals. This study thus offers a proof of principle that crystallinity responds to the action of a chemical stimulus.
A functional polymer with chemically switchable crystallinity by Jean-Christophe Daigle, Alexandre A. Arnold, Laurence Piche and Jerome P. Claverie, Polym. Chem., 2013, 4, 449-452.
Julien Nicolas is a guest web-writer for Polymer Chemistry. He currently works at Univ. Paris-Sud (FR) as a CNRS researcher.
Comments Off on Paper of the week: A functional polymer with chemically switchable crystallinity
16 Jan 2013
Chitosan (CS) is a natural polysaccharide which displays excellent biological properties such as biocompatibility, biodegradability, and antibacterial and wound-healing activity. CS has thus promising applications in many fields such as biomedicine, wastewater treatment, functional membranes and flocculation. In order to circumvent the main drawbacks of CS such as its solubility only in acid solutions and its poor mechanical properties compared to synthetic polymers, CS has been modified by chemical treatments and more efficiently by graft copolymerization. However, these methods of grafting do not generally allow controlling the molecular weight and the number of grafted chains. Controlled/living radical polymerization have therefore opened new prospects in this domain.
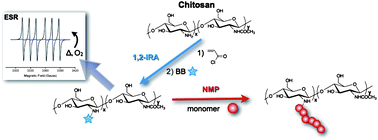
In this context, Lefay and co-workers reported an efficient CS modification method based on nitroxide-mediated polymerization (NMP) under heterogeneous conditions. After introduction of acrylamide and/or acrylate functions onto the CS backbone followed by intermolecular 1,2 radical addition of the BlocBuilder alkoxyamine, methyl methacrylate in the presence of a small amount of acrylonitrile or sodium 4-styrenesulfonate was successfully polymerized by NMP under the SG1 nitroxide control. Analyses revealed that 20-30 wt% of synthetic polymers were grafted onto the CS backbone, yielding a hybrid material with potential applications such as a biocompatibilizer.
Heterogeneous modification of chitosan via nitroxide-mediated polymerization by Catherine Lefay, Yohann Guillaneuf, Guillaume Moreira, Joel J. Thevarajah, Patrice Castignolles, Fabio Ziarelli, Emily Bloch, Mohamed Major, Laurence Charles, Marianne Gaborieau, Denis Bertin and Didier Gigmes, Polym. Chem., 2013, 4, 322-328.
Julien Nicolas is a guest web-writer for Polymer Chemistry. He currently works at Univ. Paris-Sud (FR) as a CNRS researcher.
Comments Off on Paper of the week: Heterogeneous modification of chitosan via nitroxide-mediated polymerization
11 Jan 2013
Macrocyclic non-collapsible p-conjugated structures are known to be useful in the preparation of 1D nanotubes, 2D porous networks and 3D inclusion complexes through either unique p–p stacking or concave–convex interactions. In particular for medium to giant cyclic structures, i.e. larger than 2 nm in diameter, the p-conjugated system allows benzene, thiophene, pyridine and acetylene-based macrocycles to exhibit peculiar optical, electronic, and self-assembly properties compared to their linear homologues. Structures with fully conjugated p-systems are particularly appealing because of the absence of edge effects in the p-system due to chain ends. To date, only a few examples of fully conjugated cyclic periphery have been reported. Among those, several kinds of macrocyclic oligothiophenes have attracted considerable attention since such macrocycles exhibit interesting electronic properties such as non-linear optical effects.
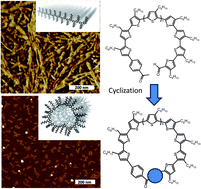
In this study, Coulembier and co-workers have demonstrated that the preparation of giant regioregular poly(3-hexylthiophene) (P3HT) cyclics is possible with a simple but unperfected aldol reaction from a pre-formed telechelic P3HT. The four-step synthetic strategy is based on a non-metallic aldol cyclization of a designed Luscombe-type regioregular P3HT. AFM analyses highlight that linear and macrocyclic P3HTs give different self-assembled nanostructures. Interestingly, the tubular assembly of the macrocyclic P3HT, with an estimated diameter of 5 nm, could be exploited to incorporate nano-sized structures such as carbon nanotubes or fullerenes, with potential application as a compatibilizer for bulk heterojunction photovoltaic diodes for instance.
Macrocyclic regioregular poly(3-hexylthiophene): from controlled synthesis to nanotubular assemblies by Olivier Coulembier, Gaelle Deshayes, Mathieu Surin, Julien De Winter, Florian Boon, Cecile Delcourt, Philippe Leclere, Roberto Lazzaroni, Pascal Gerbaux and Philippe Dubois, Polym. Chem., 2013, 4, 237-241.
Julien Nicolas is a guest web-writer for Polymer Chemistry. He currently works at Univ. Paris-Sud (FR) as a CNRS researcher
Comments Off on Paper of the week: Macrocyclic regioregular poly(3-hexylthiophene)




















