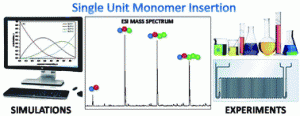
Haven et al. describe the efficiency of single monomer insertion via both kinetic simulations and experimental observations.
So-called sequence controlled materials have recently received considerable interest due to the precise and freely selectable order of monomers in a monodisperse chain. Such materials exhibit the precision of the peptides in all aspects and differentiate this approach from the synthesis of multiblock copolymers, where a significant dispersity (albeit <1.10 in many occasions) is displayed. Herein, Junkers and co-workers provide an in depth elucidation of the crucial factors that should be taken into account when performing single unit monomer insertion (SUMI) reactions. Both modelling and experimental data confirm that isolated yields of each insertion are comparatively low when going beyond the third monomer addition and as such, even lower yields must be expected for further monomer insertions. Kinetic simulations have shown that most reaction conditions play only a minor role for the success of the insertions and thus, a wide range of conditions can be applied for the synthesis of such materials. Moreover, the effect of the chain-length dependency on the SUMI reactions has also been critically evaluated. Importantly, the carefully optimized conditions obtained from microreactor experiments and kinetic modelling has been subsequently applied to upscale the SUMI reactions in a mesoflow reactor. Although the facile access to such materials demonstrates the pathway towards future developments in the synthesis of longer sequence controlled oligomers, the challenge remains whether oligomers with chain length above 5 will also be available
Tips/comments directly from the authors:
- For Single Unit Monomer Insertion reactions (SUMIs), product yield optimization is by stopping the reaction after exactly one monomer equivalent consumption. The reaction rate, thus radical initiator concentration, temperature and overall monomer conversions play a minor role; SUMIs can thus be performed within few minutes.
- To study the yield of a SUMI reaction, one needs to distinguish isolated yield from the yield in the crude product mixture. Practically, isolated yields are very dependent on the efficiency of the product isolation method. Yields from the crude can be obtained by careful calibration of mass spectra intensities.
- As long as monomers with more or less equal reactivities are chosen, a yield of ~50% is the theoretical maximum.
- Evaluation of experimental yields under optimized conditions show that the yield decreases with increasing length of the sequence-defined oligomers. This effect is attributed to a strong chain-length dependency of the monomer propagation rate coefficients.
- For upscaling of SUMI reactions, micro- and mesoflow reactors offer the perfect solution.
Efficiency assessment of single unit monomer insertion reactions for monomer sequence control: kinetic simulations and experimental observations, by J.J. Haven, J. Vandenbergh, R. Kurita, J. Gruber and T. Junkers, Polym. Chem., 2015, 6, 5752-5765.
Dr. Athina Anastasaki is a Web Writer for Polymer Chemistry. She is currently a Warwick (UK)/ Monash (Australia) research fellow working under the Monash Alliance. Visit http://haddleton.org/group-members for more information.