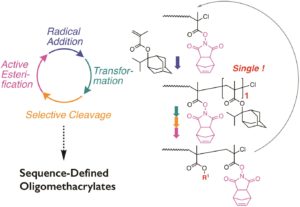
Being able to achieve perfect sequence of the repeating units/monomers has recently attracted significant attention within the polymer chemistry community and the ultimate aim is to achieve similar monomer precision with natural biomacromolecules, such as DNA or proteins. Ouchi and co-workers contributed to this direction by exploring in detail the iterative single unit monomer radical addition of a bulky tertiary, adamantyl and isopropyl methacrylic monomer (IPAMA) in order to yield sequence-defined oligo- or poly(methacrylates) in high yields. The authors focused on improving the efficiency of the single unit addition and eliminating all unfavourable products, which is a significant challenge of this technique. To achieve this, the introduction of an activated ester for the alkyl halide or the adduct was essential in improving the accuracy of the single unit addition of IPAMA. In particular, a 4 step cycle consisting of “radical addition”, “transformation”, “selective cleavage” and “active esterification” was elegantly developed. Although one more step was required to change the electron density of the halogen terminal, the efficiency of single unit addition was enhanced and high yields were obtained. Importantly, and despite the yields being close to 100%, the authors suggest that the additional introduction of some supporter such as solid resin would make the presented approach much more scalable and practical.
Tips/comments directly from the authors:
- The adamantyl and isopropyl methacrylic monomer (IPAMA) shows no homopolymerization ability due to the bulkiness. The double bond of IPAMA is active enough for radical species like general methacrylic monomers and thus single unit addition is anticipated under the condition of reversible deactivation radical polymerization or controlled radical polymerization.
- The tertiary and bulky ester pendant can be transformed into less bulky alkyl substituent. Such transformation allows iterative single unit addition to give sequence-defined methacrylic oligomers and polymers.
- The introduction of an activated ester in an alkyl halide dormant species for ATRP allows quantitative single unit radical addition of IPAMA, in contrast to general alkyl halide resulting in bimolecular coupling and/or less quantitative reaction.
- The activated ester pendant on the penultimate unit for the adduct is transformed into alkyl ester (transformation), followed by selective cleavage of the terminal IPAMA unit under acidic condition. The relative high molecular weight of N-hydroxy-5-norbornene-2,3-dicarboxyimide ester as activated ester was useful for study in single unit addition by SEC.
- The excess amount of IPAMA (10 eq for the halide) is required to complete the radical addition at a suitable rate. The unreacted monomer can be removed by preparative SEC or silica column chromatography.
- The Cp*-based ruthenium complex with bisphosphine monoxide was useful as the catalyst for single unit radical addition. The copper catalyst with dNbpy was also available. Other copper catalysts were not studied. The high efficiency in the radical addition is desirable for synthesis of sequence-defined methacrylic oligomers and polymers in high yields.
- Temperature is important to balance the quantitative reaction with the speed.
- COMU was best among condensing agents for the esterification studied in this work.
Read the full article for FREE until 3rd June!
Precise control of single unit monomer radical addition with a bulky tertiary methacrylate monomer toward sequence-defined oligo- or poly(methacrylate)s via the iterative process, Polym. Chem., 2019, 10, 1998-2003, DOI: 10.1039/C9PY00096H
About the webwriter
Dr. Athina Anastasaki is an Editorial Board Member and a Web Writer for Polymer Chemistry. Since January 2019, she joined the Materials Department of ETH Zurich as an Assistant Professor to establish her independent research group.