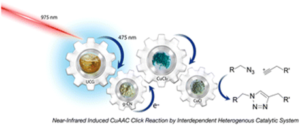
Kocaarslan et al. employ a heterogeneous near-infrared catalytic system using up-conversion glass (UCG) and g-CN to synthesize (macro)molecules via click chemistry.
The efficiency and accessibility of “click” chemistry, and more specifically the copper-catalyzed azide-alkyne cycloaddition reaction (CuAAC), have been valorized in synthetic macromolecular chemistry via a plethora of approaches. In this synthetic arena, photochemical process have been shown to efficiently achieve the in situ reduction of air-stable copper(II) species to the active copper(I) species catalyzing CuAAC.
Expanding the scope of current CuAAC photocatalysis, Yagci and coworkers developed a “geared photochemistry” approach for NIR induced CuAAC click chemistry using a dual-heterogeneous photocatalyst that generates light emission in upconversion materials combined with graphitic carbon nitride (g-CN). To achieve this, it is shown that Tm3+ and Yb3+ ion-doped zinc-tellurite glass that absorbs laser irradiation at 975 nm and is capable of emitting blue light at 475 nm, can photocatalytically activate g-CN via an internal light emitting process. For the CuAAC process, this visible light excitation of g-CN in the presence of CuCl2/PMDETA was proven to generate active copper(Ι) species able to catalyze a CuAAC click reaction between various azide and alkyne compounds. This system was proven efficient in click reactions between macromolecular chains such as azide functional polystyrene (PS-N3) and alkyne functional poly(ε-caprolactone) (PCL-alkyne) yielding block copolymers with structurally different segments. In the same vein, photoinduced crosslinking could also be achieved upon irradiation of multifunctional click components (such as bisphenol A di(3-azido-2-hydroxy propan-1-ol) ether and 1-(prop-2-yn-1-yloxy)-2,2-bis((prop-2-yn-1yloxy)methyl) butane) with a 875 nm laser in the presence of mesoporous graphitic carbon nitride (mpg-CN) and CuCl2/PMDETA under open air conditions within 2 hours. Importantly, the heterogeneous catalyst prepared via the combination of graphitic carbon and UCG could be successfully used several times enhancing the applicability of the system.
The interdependent heterogeneous system using UCG in conjunction with g-CN under NIR light presented in this study, offers a highly efficient click methodology for (macro)molecules in synthetic (polymer) chemistry.
Tips/comments directly from the authors:
- Our group’s research activities focus on the development of new photoinitiating systems for macromolecular synthesis. In this line, many photoinitiators acting at wide wavelength range of the electromagnetic spectrum were developed. “Geared photochemistry”, introduced for the first time in this paper, reflects the light-triggered reaction sequence that interdependently proceeds . In this approach, up-conversion glass absorbs light at NIR region and convert it to visible light. Upon absorption of the emitted visible light graphitic carbon nitride (mpg-CN) in the reaction media creates electron and hole pairs. The copper (II) complex, which has no absorbance at these two wavelengths, is reduced from copper II to copper I by the released electrons. After all this gear-like system, copper I ions catalyze the click reaction between azide and alkyne compounds to form a triazole ring. We are happy to publish this work in an important journal in polymer science, Polymer Chemistry.
- This approach will open new horizons not only for click chemistry, but also for many synthesis procedures involving electron transfer reactions. It should be considered that the change of absorbance with upconversion glasses is important for many light-activated photocatalysts.
Citation to the paper: Geared photochemistry: an interdependent heterogeneous near-infrared catalytic system using up-conversion glass and g-CN for CuAAC chemistry, Polym. Chem., 2022,13, 6393-6399, DOI: 10.1039/D2PY01075E
Link to the paper:
https://pubs.rsc.org/en/content/articlelanding/2022/py/d2py01075e
Kelly Velonia is saddened to hear about the passing of Prof. Yusuf Yagci, an exceptional scientist and person. Condolences to his family and loved ones. The polymer community will certainly miss him.
 |
Dr. Kelly Velonia is an Advisory Board Member and a Web Writer for Polymer Chemistry. She joined the Department of Materials Science and Technology at the University of Crete in 2007. Research in her group focuses on the synthesis and applications of bioconjugates and biopolymers. You can follow Kelly on twitter @KellyVelonia
|