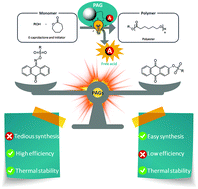
Sardon’s group investigate the ring opening polymerization of cyclic esters using novel photo acid generators.
Light has emerged as a powerful stimulus allowing for spatial and temporal control over polymerization kinetics, macromolecular sequence, and composition and has enabled a number of high-end applications including coatings, microelectronics, additive manufacturing and 3D printing. However, the potential of light in polymer chemistry is far from being fully exploited. Photopolymerization is currently dominated by (controlled) radical polymerizations of vinyl monomers. Little attention has been paid to photo-induced cationic ring-opening polymerization (CROP) of cyclic esters. In a recent contribution to Polymer Chemistry, Sardon and co-workers developed six new photocatalysts for light-mediated CROP. Upon exposure to light, the new photocatalysts release strong sulfonic acids that can trigger the CROP of ε-caprolactone. The authors particularly focused on imino-sulfonates and aryl-sulfonates based photocatalysts, and this strategy was hugely successful. Complete monomer conversion was obtained after only 5 minutes of irradiation. This is an impressively high polymerization rate despite the catalyst efficiency being typically strongly related to the chromophore and the sulfonate substituent. In addition, several of these photocatalysts are stable even at 100 °C and were successfully used to produce not only linear biodegradable polymer polymers but also crosslinking poly(ε-caprolactone) exhibiting excellent mechanical properties. Furthermore, the authors employed density functional theory calculation to propose a photodissociation mechanism. The studied photopolymerization has also been successfully applied to surface coating. The potential applications of these new photocatalysts are certainly not limited to photocoating, and therefore, we look forward to seeing further exciting applications of these photocatalysts.
Tips/comments directly from the authors:
- The efficiency of the PAGs was observed to be highly dependent on the chromophore and the photolabile bond; imino-sulfonates were more capable of producing sulfonic acids than aryl-sulfonates. However, their preparation is tedious and requires a multistep synthesis process. Aryl-sulfonates are not as efficient but their synthesis is performed in a single step in excellent yields up to 90%.
- Imino-sulfonate based photocatalyst were able to promote the ring opening polymerization of ε-caprolactone at room temperature but 3 h were required for getting full conversion. Nevertheless, as several of these catalyst were stable up to 100 °C we were able to get full conversion in just 5 minutes at 100 °C
- To further expand the applications of the studied PAGs, we demonstrated the ability of the photoacid to promote the crosslinking at room temperature in the presence of a dilactone. While the crosslinking reaction was successful, long reaction times were required for reaction completion, making impractical the use of these photocatalyst in 3 D printing applications.
Citation to the paper: Novel imino- and aryl-sulfonate based photoacid generators for the cationic ring-opening polymerization of ε-caprolactone, Polym. Chem., 2021,12, 4035-4042, DOI: 10.1039/D1PY00734C
Link to the paper:
https://pubs.rsc.org/en/content/articlelanding/2021/py/d1py00734c#!divAbstract
 Dr. Athina Anastasaki is an Editorial Board Member and a Web Writer for Polymer Chemistry. Since January 2019, she joined the Materials Department of ETH Zurich as an Assistant Professor to establish her independent research group.
Dr. Athina Anastasaki is an Editorial Board Member and a Web Writer for Polymer Chemistry. Since January 2019, she joined the Materials Department of ETH Zurich as an Assistant Professor to establish her independent research group.