 Zhang and co-workers report aqueous polymerization-induced self-assembly by atom transfer radical polymerization to generate protein-based nanoassemblies.
Zhang and co-workers report aqueous polymerization-induced self-assembly by atom transfer radical polymerization to generate protein-based nanoassemblies.
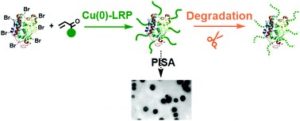
Polymerization-induced self-assembly (PISA) has opened the way for the in-situ formation of a wide range of nanoparticles with applications ranging from the material to the biomedical field. However, the vast majority of reports focus on utilizing reversible addition-fragmentation chain transfer polymerization as the main methodology while atom transfer radical polymerization (ATRP) is very rarely combined with PISA, mostly due to the limitations of ATRP in water. Zhang and co-workers utilized Cu(0) reversible deactivation radical polymerization by exploiting the disproportionation of CuBr/ligand in aqueous media generating both Cu(0) particles and Cu(II) deactivator. Upon modifying Candida Antarctica lipase B (CALB), it was used as a macroinitiator for both hydrophilic and hydrophobic monomers generating well-defined protein-based nanoassemblies. A range of acrylamide and acrylate based monomers were successfully polymerized under mild reaction conditions (e.g. room temperature) via he “grafting from” strategy. When hydrophilic monomers were selected, water-soluble conjugates could be obtained in a facile manner while by polymerizing more hydrophobic monomers yields spherical nanoparticles, consistent to a traditional PISA formulation. Importantly, it was also found that they hydrolysis of the ester bonds can be very significant in the presence of lipase-based macroinitiators, which will catalyze the hydrolysis of poly(acrylate) to poly(acrylic acid). The versatility of the reported methodology combined with the use of mild reaction conditions may find applications in enzyme immobilization and nanoreactors.
Tips/comments directly from the authors:
- It is necessary to purify the commercial CuBr as it could be partially oxidized during storage and routine use.
- Typical Cu(0)-RDRP in water is fast enough to reach full conversion in minutes; however, the polymerizations would be slower when grafting from proteins, possibly due to the low concentration of macroinitiators.
- Although copper ions were known to be able to denature proteins, CALB still maintained its function after polymerization. The mild reaction conditions such as aqueous system, low reaction temperature (0-25 ℃) and fast polymerization rate (minutes to hours) could be suitable for more sensitive proteins.
- The degradation of lipase-poly(acrylate) conjugates was fast and gradual disappearance of precipitates could even be visually observed during the dialysis in water. So it is better to quickly purify the conjugates via centrifugation. From another point of view, such conjugates could be potentially used for drug delivery and controlled release.
Citation to the paper: Synthesis of lipase–polymer conjugates by Cu(0)-mediated reversible deactivation radical polymerization: polymerization vs. degradation, Polym. Chem., 2020, 11, 1386-1392, DOI: 10.1039/c9py01462d
Link to the paper:
https://pubs.rsc.org/en/content/articlelanding/2020/py/c9py01462d#!divAbstract
This paper is free to read until 10th April 2020!
About the Web Writer
Dr. Athina Anastasaki is an Editorial Board Member and a Web Writer for Polymer Chemistry. Since January 2019, she joined the Materials Department of ETH Zurich as an Assistant Professor to establish her independent research group.