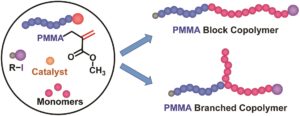
The use of a polymethylmethacrylate (PMMA) containing an unsaturated chain end as a macroinitiator during reversible complexation mediated polymerization has been previously reported by Goto and coworkers. Typically, such macroinitiators can also be used as macromonomers to generate branched polymers via propagation. In this work, Goto and co-workers elegantly demonstrate that the occurrence of addition-fragmentation chain transfer and propagation strongly depends on the temperature during the polymerization of styrene. Through carefully monitoring the kinetics of the polymerization of styrene, the authors discovered that propagation is predominant below 60 ̊C, consistent with previous reports. However, upon elevating the temperature (e.g. 120 ̊C), addition-fragmentation chain transfer dominates instead. This discovery then allowed access to the efficient synthesis of block copolymers with PMMA and polystyrene at high temperatures. Importantly, addition-fragmentation chain transfer was also predominant over propagation during the polymerizations of acrylonitrile and acrylates yielding well-defined block copolymers. PMMAs with different molecular weights were also investigated and the polymerization was controlled utilizing iodine transfer polymerization for styrene and reversible complexation mediated polymerization for the other monomers. Such an approach is highly advantageous due to the ease of the operation and it is expected to be a practical alternative for efficient block copolymer synthesis.
Tips/comments directly from the authors:
- The proper purification of polymers and the careful NMR analysis were important for obtaining the accurate kinetic data. The kinetic study provided a useful idea enabling the synthesis of block copolymers of PMMA with polystyrene (PSt).
- Block copolymers of PMMA with PSt, polyacrylonitrile, and polyacrylates are accessible. Relatively high monomer conversions are achievable.
- Not only the isolated alkyl iodide but also the alkyl iodide in situ generated from iodine (I2) and azo compound can effectively be used as the initiating dormant species. The in situ method is less expensive and robust and hence can be a practically attractive
Read the full article now for FREE until 10th January!
Synthesis of block copolymers using poly(methyl methacrylate) with unsaturated chain end through kinetic studies, Polym. Chem., 2019, 10, 5617-5625, DOI: 10.1039/c9py01367a
About the web writer
 Dr. Athina Anastasaki is an Editorial Board Member and a Web Writer for Polymer Chemistry. Since January 2019, she joined the Materials Department of ETH Zurich as an Assistant Professor to establish her independent research group.
Dr. Athina Anastasaki is an Editorial Board Member and a Web Writer for Polymer Chemistry. Since January 2019, she joined the Materials Department of ETH Zurich as an Assistant Professor to establish her independent research group.