 Bumjoon Kim is a Professor in the Department of Chemical and Biomolecular Engineering at KAIST since 2008 where he is appointed as the KAIST Endowed Chair Professor. He completed his doctorate under the guidance of Prof. Edward Kramer at UCSB. Then, he worked with Prof. Jean Fréchet at UC Berkeley. His research interests include development of block copolymer-based functional materials including shape-tunable particles, colorimetric sensors, and design of new electroactive polymers for all-polymer solar cells with high stability. He has published more than 170 peer-reviewed papers and 50 issued/pending patents. He was appointed as the Ewon Assistant Professor at KAIST (2010-2013). Also, he received the KAIST Academic Excellence Award (2015) and Shimgye Science Award (2017), and he was selected as 2013 Young Scientist by the World Economic Forum (DAVOS Forum) and appointed as the KAIST Endowed Chair Professor (2018). He currently serves as an editorial advisory board member of Macromolecules, ACS Macro Letters, Chemistry of Materials (ACS), J. Mater. Chem. A (RSC) and BMC Energy (Springer Nature).
Bumjoon Kim is a Professor in the Department of Chemical and Biomolecular Engineering at KAIST since 2008 where he is appointed as the KAIST Endowed Chair Professor. He completed his doctorate under the guidance of Prof. Edward Kramer at UCSB. Then, he worked with Prof. Jean Fréchet at UC Berkeley. His research interests include development of block copolymer-based functional materials including shape-tunable particles, colorimetric sensors, and design of new electroactive polymers for all-polymer solar cells with high stability. He has published more than 170 peer-reviewed papers and 50 issued/pending patents. He was appointed as the Ewon Assistant Professor at KAIST (2010-2013). Also, he received the KAIST Academic Excellence Award (2015) and Shimgye Science Award (2017), and he was selected as 2013 Young Scientist by the World Economic Forum (DAVOS Forum) and appointed as the KAIST Endowed Chair Professor (2018). He currently serves as an editorial advisory board member of Macromolecules, ACS Macro Letters, Chemistry of Materials (ACS), J. Mater. Chem. A (RSC) and BMC Energy (Springer Nature).
What was your inspiration in becoming a polymer chemist?
I’m not sure what would be the best way to simply describe it. I was educated both as a chemical engineer and a polymer scientist, not a hard core chemist. During my senior year in college, I first learned about polymers in a class named “Introduction to Polymer Engineering” taught by Prof. Kookheon Char at Seoul National University. This course gave me a strong interest in polymer science and motivated me to study polymer science further. Also, I believe I was very fortunate to receive a well-balanced education in both polymer physics and chemistry during my time at graduate school and postdoctoral studies from Profs. Edward Kramer, Craig Hawker (UCSB), and Jean Fréchet (UC Berkeley). These interests and backgrounds have led me to pursue the development of new polymer-based materials that may benefit our lives and society.
What was the motivation behind your most recent Polymer Chemistry article?
Particle shape is one of the most fundamental and essential features that determines the function of polymeric particles. For example, anisotropically shaped particles can exhibit unique optical properties, packing structures, and rheological behaviors. For the last few years, our group has made contributions to the development of the principles dictating the shape of block copolymer (BCP) particles from the evaporative emulsions. For example, I would like to highlight the papers including JACS 2014, 9982; Adv. Funct. Mater. 2018, 1802961; Macromolecules 2019, 1150. This understanding is synergistically combined with a technique called “membrane emulsification” to produce the particles of the same size and shape in a large batch (also see ACS Nano 2017, 2133; Chem. Mater. 2018, 6277), which enables the use of these particles for the various applications described above. In this Polymer Chemistry article, we describe the development of a series of non-spherical Janus particles with interesting cone-shapes. Systematic control of particle shape is achieved by programming the phase-separation of the blend of BCP and newly-synthesized copolymers confined in emulsion droplets. We also have developed a theoretical model to explain the shape of the cone-shaped particles.
Which polymer scientist are you most inspired by?
As I mentioned in the answer to the first question, people who are world-leading polymer scientists as well as great teachers have inspired me and helped a lot to develop my career and do my current research. In particular, I have learned a lot from Prof. Edward Kramer, who was my Ph.D. advisor, on many aspects, such as how to conduct research and attitudes towards students. I believe his patience and inspiration for science have greatly influenced my choice of work as a teacher and professor.
How do you spend your spare time?
I like traveling and walking/driving near the seaside. I spend enough time relaxing and refreshing outside the office. This helps me to think more clearly. Also, I enjoy spending time with my 7-year-old daughter, Jaeyeon.
What profession would you choose if you weren’t a chemist?
I would like to be a teacher or a cook. Since I like teaching, I would spend time teaching young students if I were not a chemist. Also, I like cooking, so I think becoming a cook can be a great choice for me. I think there are many connections between cooking and doing experiments.
Read his Polymer Chemistry article for FREE until 15th July
Shape control of nanostructured cone-shaped particles by tuning the blend morphology of A-b-B diblock copolymers and C-type copolymers within emulsion droplets
Eun Ji Kim, Jae Man Shin, YongJoo Kim, Kang Hee Ku, Hongseok Yun and Bumjoon J. Kim
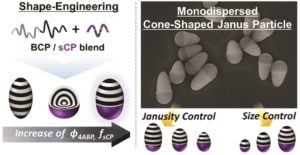
Block copolymers (BCPs) under colloidal confinement can provide an effective route to produce non-spherical particles. However, the resulting structures are typically limited to spheroids, and it remains challenging to achieve a higher level of control in the particle shape with different symmetries. Herein, we exploit the blend of BCPs and statistical copolymers (sCPs) within emulsion droplets to develop a series of particles with different symmetries (i.e. Janus-sphere and cone-shaped particles). The particle shape is tunable by controlling the phase behavior of the polymer blend consisting of a poly(styrene-block-1,4-butadiene) (PS-b-PB) BCP and a poly(methylmethacrylate-statistical-(4-acryloylbenzophenone)) (P(MMA-stat-4ABP)) sCP. A key strategy for controlling the phase separation of the polymer blend is to systematically tune the incompatibility between the BCP and sCP by varying the composition of the sCPs (ϕ4ABP, mole fraction of 4ABP). As a result, a sequential morphological transition from a prolate ellipsoid, to a Janus-sphere, to a cone-shaped particle is observed with the increase of ϕ4ABP. We further demonstrate that the shape-anisotropy of cone-shaped particles can be tailored by controlling the particle size and the Janusity, which is supported by quantitative calculation of the particle shape-anisotropy from the theoretical model. Also, the importance of the shape control of the cone-shaped particles with high uniformity in a batch is demonstrated by investigating their coating properties, in which the deposited coating pattern is a strong function of the shape-anisotropy of the particles.
About the webwriters
 Simon Harrisson is a Chargé de Recherche at the Centre National de la Recherche Scientifique (CNRS), based in the Laboratoire des IMRCP in Toulouse. His research seeks to apply a fundamental understanding of polymerization kinetics and mechanisms to the development of new materials. He is an Advisory Board member for Polymer Chemistry. Follow him on Twitter @polyharrisson
Simon Harrisson is a Chargé de Recherche at the Centre National de la Recherche Scientifique (CNRS), based in the Laboratoire des IMRCP in Toulouse. His research seeks to apply a fundamental understanding of polymerization kinetics and mechanisms to the development of new materials. He is an Advisory Board member for Polymer Chemistry. Follow him on Twitter @polyharrisson

Professor Athina Anastasaki is an Editorial Board Member and a Web Writer for Polymer Chemistry. In January 2019 she joined the Materials Department of ETH Zurich as an Assistant Professor to establish her independent research group.
 Dr. Athina Anastasaki is an Editorial Board Member and a Web Writer for Polymer Chemistry. Since January 2019, she joined the Materials Department of ETH Zurich as an Assistant Professor to establish her independent research group.
Dr. Athina Anastasaki is an Editorial Board Member and a Web Writer for Polymer Chemistry. Since January 2019, she joined the Materials Department of ETH Zurich as an Assistant Professor to establish her independent research group.















![c9py00213h-ga[1]](https://blogs.rsc.org/py/files/2019/06/c9py00213h-ga1-300x124.jpg)