Sandra Schlögl studied Technical Chemistry at Graz University of Technology (Austria), where she obtained her Master degree in 2006. In 2006, she joined the Polymer Competence Center Leoben (PCCL), which is the leading ‘Center of Excellence’ for cooperative research in the area of polymer engineering and sciences in Austria. Two years later, she received her Ph.D. degree in Polymer Chemistry from Graz University of Technology. She was a visiting scientist at Politecnico di Torino (Italy) in the group of Prof. Marco Sangermano in 2016, and finished her habilitation (post-doctoral lecturing qualification) in Macromolecular Chemistry in 2017. Currently, she heads the ‘Chemistry of Functional Polymers’ division at PCCL. In addition, she is a lecturer at Montanuniversitaet Leoben (Austria) teaching courses at an MSc level in polymer photochemistry and in stimuli-responsive polymer materials. Her research centers on stimuli-responsive polymers, dynamic networks, elastomer chemistry and photochemistry in polymers. She is author of more than 50 peer-reviewed publications (Scopus H-index of 10), inventor of 11 patents (national and international) and has received several awards for her research (e.g. Paul Dufour Award in 2015, EARTO Innovation Award in 2016).
Sandra Schlögl studied Technical Chemistry at Graz University of Technology (Austria), where she obtained her Master degree in 2006. In 2006, she joined the Polymer Competence Center Leoben (PCCL), which is the leading ‘Center of Excellence’ for cooperative research in the area of polymer engineering and sciences in Austria. Two years later, she received her Ph.D. degree in Polymer Chemistry from Graz University of Technology. She was a visiting scientist at Politecnico di Torino (Italy) in the group of Prof. Marco Sangermano in 2016, and finished her habilitation (post-doctoral lecturing qualification) in Macromolecular Chemistry in 2017. Currently, she heads the ‘Chemistry of Functional Polymers’ division at PCCL. In addition, she is a lecturer at Montanuniversitaet Leoben (Austria) teaching courses at an MSc level in polymer photochemistry and in stimuli-responsive polymer materials. Her research centers on stimuli-responsive polymers, dynamic networks, elastomer chemistry and photochemistry in polymers. She is author of more than 50 peer-reviewed publications (Scopus H-index of 10), inventor of 11 patents (national and international) and has received several awards for her research (e.g. Paul Dufour Award in 2015, EARTO Innovation Award in 2016).
What was your inspiration in becoming a polymer chemist?
My career as a polymer chemist was not planned. In school, I always had a talent for natural-science subjects and back then, as today, I was intrigued by biological and biochemical processes of the human body. Obviously, my first plan was to study medicine and I even took Latin courses at school. However, during my voluntary work in the health care sector, I recognized that medicine is interesting in theory but hard to carry out in practice. I started to rethink my career plans, which led to my decision to study chemistry. The curriculum of the technical chemistry studies sounded promising since it also contained biochemistry, which was not so far away from medicine. However, during my studies I developed a favorite research activity: creating functional polymers. I took inspiring lectures and lab courses in polymer photochemistry, where I learned versatile and creative routes to change material properties on demand. Since then, the chemistry of polymers has me hooked.
What was the motivation behind your recent Polymer Chemistry article?
A strong focus of my working group is the synthesis of stimuli-responsive polymers, by introducing photocleavable chromophores and photoreversible binding motifs into polymer structures. These polymers change their material characteristics in response to external stimuli (such as light and temperature), which is used for numerous applications such as self-healable materials, reversible adhesives or switchable micropatterns.
Since several years, I have successfully cooperated with Marco Sangermano and Ignazio Roppolo from Politecnico di Torino on several research topics, joining the expertise of our working groups. Ignazio is not only a dedicated soccer player but also watches documentaries in his spare time. He was fascinated by the ability of desert beetles to collect humidity and transport water droplets across their skin. We discussed the topic and the idea was born to transfer the concept to photopolymer materials. Mimicking nature with synthetic materials for controlled water transport is not new, but the majority of the reported concepts rely on inorganic materials requiring time consuming and elaborate sample preparation techniques. With our recent Polymer Chemistry article, we succeeded to generate multi-gradients on polymer surfaces simply by light exposure. We introduced both a wettability gradient and a Laplace pressure gradient by a localized light-induced switching of the polarity, which is required to drive a water droplet across the photopolymer surface in a controlled way. This opens the path towards a precise movement of individual or multiple droplets on surfaces with complex topology and tailored surface polarity.
Which polymer scientist are you most inspired by?
In my field of research I am most inspired by the pioneering work of two scientists: Christopher N. Bowman (University of Colorado Boulder) and Christopher Barner-Kowollik (Queensland University of Technology). They are at the forefront of research in polymer photochemistry and their research is an important driving force for the development of new photopolymers with fascinating properties.
Can you name some up and coming polymer chemists who you think will have big impact on the field?
In my opinion, the research work of Miriam M. Unterlass (Vienna University of Technology) on hydrothermal polymerization has the potential to greatly influence future synthesis routes in polymer chemistry. Her research focuses on geomimetics, which takes inspiration from nature for generating novel synthetic materials under high pressure and with water as solvent. In one-pot reactions with low energy consumption, she is able to synthesis a great variety of materials involving polymers, dyes or inorganic-organic hybrids. I think that Miriam’s research has a great potential for the ‘green’ synthesis of new and traditional high-performance polymers; not only on a lab but also on an industrial scale.
How do you spend your spare time?
I am fond of outdoor sports, which are a good balance to my professional life, which over the past years is less and less taking place in the chemical lab but more in the office. I am a hobby racing cyclist, who tries to avoid the mountain areas, which is not an easy task in Austria and I do some running in between. I also enjoy spending time with my family and friends and in case of bad weather I like reading novels from different genres.
What profession would you choose if you weren’t a chemist?
I would choose again a profession in science since I can only agree with Madame Marie Curie that ‘science has great beauty’.
Read her Polymer Chemistry article for FREE until June 10th!
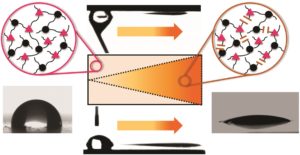
Directed motion of water droplets on multi-gradient photopolymer surfaces
The present work highlights the controlled directional movement of water droplets across a photopolymer surface. The movement is propelled by multi-gradients including a wettability gradient and a Laplace pressure gradient. Both gradients are conveniently adjusted by light employing a photoresponsive thiol–yne photopolymer. o-Nitrobenzyl alcohol derivatives with terminal alkyne groups are synthetized and cured across di- and tri-functional thiols upon visible light exposure. The wettability gradient is generated in a subsequent step involving an asymmetrical irradiation of the polymer surface with light in the UV-A spectral region. Polar groups are formed in the exposed areas due to the photocleavage of the chromophore and photo-oxidation reactions (upon prolonged UV exposure in air). The wettability rises with increasing exposure dose and gradient surfaces are prepared with static water contact angles ranging from 97 to 19°. By simultaneously inscribing the wettability gradient in wedge-shaped patterns, a Laplace pressure gradient is realized on the photopolymer surface, which can be easily tailored by the size and the angle of the wedge. The combination of both gradients enables a rapid and directed movement of water droplets (2 μL droplet) over a reasonable distance (up to 10 mm). Due to the high adhesion of the photopolymer surface, the droplet can be driven in a controlled way, even if the surface is inclined (20°) or turned upside down.
About the Web writer
 Simon Harrisson is a Chargé de Recherche at the Centre National de la Recherche Scientifique (CNRS), based in the Laboratoire des IMRCP in Toulouse. His research seeks to apply a fundamental understanding of polymerization kinetics and mechanisms to the development of new materials. He is an Advisory Board member for Polymer Chemistry. Follow him on Twitter @polyharrisson
Simon Harrisson is a Chargé de Recherche at the Centre National de la Recherche Scientifique (CNRS), based in the Laboratoire des IMRCP in Toulouse. His research seeks to apply a fundamental understanding of polymerization kinetics and mechanisms to the development of new materials. He is an Advisory Board member for Polymer Chemistry. Follow him on Twitter @polyharrisson