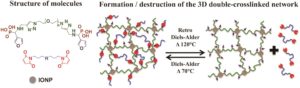
Double-network hydrogels (DN gels) consist of two interpenetrating networks that are bound together by covalent and reversible interactions and can resist high deformation by reorganizing their structure. Magnetic hybrid hydrogels in particular have attracted considerable attention owing to the possibility of triggering the properties of the hydrogels with an external magnetic field. Fontaine, Montembault and co-workers further contributed to this field by developing a novel class of thermoresponsive hybrid double-crosslinked polymer networks materials that can rearrange and rebuild upon triggering a Diels-Alder (DA) reaction. Central to this approach is the use of iron oxide nanoparticles as the nano-crosslinkers and difuran-functionalized poly(ethylene oxide) as the diene partner for the thermoreversible DA reaction. The thermoreversibility of the network was confirmed by 1H nuclear magnetic resonance (NMR) spectroscopy and rheological studies showing a fast gel/liquid state transition upon heating the sample. Importantly, the rheological properties of 3D networks with and without iron oxide nanoparticles were compared. The studies conclude that the presence of iron oxide nanoparticles in the network ensured that a gel-like structure was maintained after the retro DA reaction. These characteristics were attributed to the establishment of a secondary network through covalently integrated iron oxide nanoparticles. The unique combination of the Diels-Alder reaction with iron oxide nanoparticles to generate new reversible reticulated networks can pave the way for further applications mediated by magnetic hyperthermia stimuli.
Tips/comments directly from the authors:
1. The strategy used for the synthesis of difuran-functionalized diphosphonic acid terminated poly(ethylene oxide) by a combination of Kabachnik-Fields reaction and “click” copper-catalyzed 1,3-dipolar cycloaddition is a versatile method and poly(ethylene oxide) backbone can be replaced by a wide range of polymers that may bring new properties.
2. The formation of a 3D network via the Diels-Alder (DA) reaction with a trismaleimide is thermoreversible, with a faster retro-DA (rDA) reaction rate compared to the DA reaction, leading to an easier destruction of the 3D network than its formation.
3. The presence of phosphonic acid groups is needed to allow the crosslinking through interactions with the iron oxide nanoparticles and the formation of the 3D double-crosslinked network.
4. The 3D network is preserved even after the rDA reaction, demonstrating that the iron oxide nanoparticles serve as crossing points through strong bidendate Fe-O-P bonds. Furthermore, a gel-like structure is maintained at least in the limit of the percolation threshold.
5. The viscoelastic properties of the double-crosslinked gels demonstrates that the double-crosslinking leads to stiffer gels.
Read the full article for FREE until 26th November!
Thermoresponsive hybrid double-crosslinked networks using magnetic iron oxide nanoparticles as crossing points, Polym. Chem., 2018, 9, 4642-4650, DOI: 10.1039/C8PY01006D
About the web writer
 Dr. Athina Anastasaki is a Web Writer for Polymer Chemistry. She is currently a Global Marie Curie Fellow working alongside Professor Craig Hawker at the University of California, Santa Barbara (UCSB). In January 2019, she will join the ETH Materials Department as an Assistant Professor to establish her independent group.
Dr. Athina Anastasaki is a Web Writer for Polymer Chemistry. She is currently a Global Marie Curie Fellow working alongside Professor Craig Hawker at the University of California, Santa Barbara (UCSB). In January 2019, she will join the ETH Materials Department as an Assistant Professor to establish her independent group.