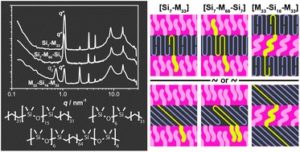
A long lasting challenge in polymer chemistry is to design new block copolymer combinations that allow a decrease of feature sizes and to propose models that describe the molecular organization within the microphase-segregated structures. A recently developed way to achieve this is through a new class of low molecular weight, discrete block copolymers (dispersity of 1). In this contribution, Meijer, Palmans and co-workers designed and synthesized a new class of discrete-length block co-oligomers comprising of oligodimethylsiloxane (oDMS) and oligomethylene (oM). By employing differential scanning calorimetry and small-angle X-ray scattering it was shown that all block co-oligomers exhibit microphase separation into well-ordered lamellar morphologies, driven by the crystallization of the oM blocks. Pre-melting order-order transitions were found for a number of block co-oligomers, resulting in an alternation of the oM crystal packing and in changes of the overall microphase-segregated structure. Importantly, uniform microphase-segregated domains were discovered and among them, one of the smallest domain spacing ever reported (dLAM=5.8 nm), highlighting that the combination of small feature sizes and structural perfection is unique for this type of materials. The authors also elegantly proposed models to describe the molecular organisation within the microphase-segregated structures. This was achieved by evaluating the changes in the lamellar thickness upon alternation of the block co-oligomer architecture. Such type of materials are of critical importance to fundamentally understand the molecular structure and the self-assembly of polymeric materials.
Tips/comments directly from the authors:
- The large difference between the affinities of oDMS hydride and oDMS with a silanol endgroup toward silica remains a useful tool to separate traces of starting material from the product during the oDMS synthesis. Secondly, the large difference in solubility of short (< 11 repeat units) and long (> 11 repeat units) siloxane oligomers was used frequently to purify the materials.
- During the synthesis of the oM blocks, we routinely used a two-stage protection and deprotection protocol of the cyclic ethylene acetal via a dialkyl acetal intermediate.
Thus, stages involving the free aldehyde could be conducted at room temperature, minimizing the risk of degradation of the aldehyde functionality, which otherwise might led to inseparable side-products (e.g., the result of unwanted condensation reactions).
- To ensure good solubility of the oM blocks, a molecular design containing at least one double bond per 30 carbon atoms is advised.
- Crystallisation kinetics in oDMS–oM and related systems generally are very fast. In a select number of cases we clearly noticed the benefits of very slow (< 0.1 °C min-1) cooling from the melt in order to decrease the number of defects/increase the size of the crystalline domains in the phase-segregated systems.
This article is free to read and download until 26 June
Discrete oligodimethylsiloxane–oligomethylene di- and triblock co-oligomers: synthesis, self-assembly and molecular organisation, Polym. Chem., 2018, 9, 2746-2758, DOI: 10.1039/c8py00355f
About the webwriter
Dr. Athina Anastasaki is a Web Writer for Polymer Chemistry. She is  currently a Global Marie Curie Fellow working alongside Professor Craig Hawker at the University of California, Santa Barbara (UCSB). Please, visit this link for more information.
currently a Global Marie Curie Fellow working alongside Professor Craig Hawker at the University of California, Santa Barbara (UCSB). Please, visit this link for more information.