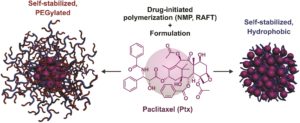
Paclitaxel (Ptx) is one of the most widely used chemotherapeutic agents for the treatment of a broad range of human tumors. Polymer prodrugs are often employed to solve a number of issues associated with its limited water solubility, the absence of ionisable groups to enable Ptx salt formation and the short colloidal stability of its formulations. In a way analogous to polymer synthesis, the “grafting from” approach, also referred to here as “drug-initiated” consists of the controlled growth of a polymer from a drug. However, this approach is limited by the poor colloidal stability of hydrophobic drug-polymer nanocarriers and the lack of the direct synthesis of PEGylated prodrugs. Nicolas and co-workers managed to tackle these issues by developing a global method which enables the facile derivatization of Ptx followed by the subsequent reversible deactivation radical polymerization to design surfactant-free, Ptx-polymer prodrug nanocarriers with contrasting properties. In particular, nitroxide-mediated polymerization (NMP) and reversible addition-fragmentation chain transfer polymerization were elegantly selected to grow short polyisoprene or poly[(oligo(ethylene glycol) methyl ether methacrylate)] chains from Ptx in a controlled fashion. This allowed for the formation of either self-stabilized, all-hydrophobic Ptx-polymer prodrug nanoparticles or their PEGylated counterparts. Importantly, these prodrug nanocarriers exhibited high cytotoxicity on three different cancer cell lines, with chain length-cytotoxicity dependency and IC50 values comparable to those of the parent drug. This versatile approach demonstrates the robustness and the broad use of the drug-initiated method for the simple design of efficient polymer prodrug nanoparticles consisting of polymers of opposite nature, thus opening new perspectives in the nanomedicine field.
Tips/comments directly from the authors:
- The drug-initiated NMP of isoprene from Ptx is a very simple yet efficient method to prepare surfactant-free, stable polymer prodrug nanoparticles with high drug payload, without any protection/deprotection chemistry.
- When using the AMA-SG1 alkoxyamine for Ptx derivatization, the resulting Ptx-AMA-SG1 alkoxyamine is obtained as a mixture of diastereomers (this is related to the two chiral centers of the alkoxyamine). The signals from the NMR spectrum should be carefully assigned. Alternatively, the diastereomers can also be separated by column chromatography with a less polar eluent.
- Mn of PEGMA-based prodrugs are higher than those of PI-based prodrugs because shorter POEGMA chains hardly precipitate compared to PI with similar Mn. Dialysis was not attempted because of potential hydrolytic cleavage between the drug and the polymer (especially with the diglycolate linker)
Self-stabilized, hydrophobic or PEGylated paclitaxel polymer prodrug nanoparticles for cancer therapy, Polym. Chem., 2018, 9, 687-698, DOI: 10.1039/C7PY01918A
This article is free to read until 16 April 2018
About the webwriter
 Dr. Athina Anastasaki is a Web Writer for Polymer Chemistry. She is currently a Global Marie Curie Fellow working alongside Professor Craig Hawker at the University of California, Santa Barbara (UCSB). Please, visit this site for more information.
Dr. Athina Anastasaki is a Web Writer for Polymer Chemistry. She is currently a Global Marie Curie Fellow working alongside Professor Craig Hawker at the University of California, Santa Barbara (UCSB). Please, visit this site for more information.