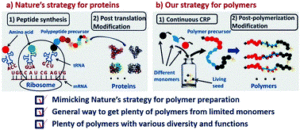
Nature is capable of synthesizing an unlimited number of biomacromolecules (e.g. proteins) with remarkable structures and functions by simply starting from only 20 amino acids. This process lies in the precise sequence-controlled polymerization of amino acids to control the primary structures of polypeptide precursors, followed by a highly efficient post-translation modification in order to define these structures.
Inspired by nature’s strategy to synthesize proteins, Tao and co-workers developed a two-stage method to synthesize a large number of polymers with precisely controlled structures, different functionalities and various molecular diversities. Key to their strategy is the combination of controlled radical polymerization and post-polymerization modification. Specifically, reversible addition-fragmentation chain transfer (RAFT) polymerization was utilized to synthesize the polymer precursors starting from only 3 acrylamide monomers. By repeating the polymerization with different monomer sequences, 6 triblock copolymers with controlled chain ends, molecular weights and molar mass distributions were obtained. The different polarity of all synthesized precursors was then confirmed by reverse-phase high performance liquid chromatography (HPLC). The triblock copolymers were subsequently modified via the Biginelli reaction to rapidly generate 60 derivatives in a high-throughput (HPT) manner. HTP analyses was also conducted as an efficient and quick way to verify specific functionalities (e.g. radical scavengers, metal chelating agents, etc.).
In summary, the authors presented an efficient strategy to prepare and characterize large libraries of polymers with diverse structures and functions.
Tips/comments directly from the authors:
1. For the Biginelli reaction, acetic acid/MgCl2 is an efficient solvent/catalyst system to smoothly get the targeted compounds. However, this system is not as efficient for aliphatic aldehydes. Fortunately, the Biginelli reaction has been studied for more than 100 years and as such, many other solvent/catalyst systems have been established in this time. Thus, people can choose different conditions to perform the Biginelli reaction for the post-polymerization modification depending on the specific requirements and applications.
2. For the high throughput analysis of radical scavengers, the oxygen in the air might also quench the radical, and the radical colour was found to fade faster in summer than in winter. Thus, the use of fresh reagents and careful recording of the temperature is recommended.
3. The ultra-fast RAFT was used in the present work as a model polymerization to prepare copolymers. The authors believe other advanced controlled radical polymerization techniques (SET-ATRP, photo-induced CRPs, sulfur-free RAFT, etc.) can also be used to prepare multiblock copolymers, especially when thermo-sensitive monomers are used.
Polymer synthesis by mimicking nature’s strategy: the combination of ultra-fast RAFT and the Biginelli reaction, Polym. Chem., 2017, 8, 5679-5687, DOI: 10.1039/c7py01313b
Dr. Athina Anastasaki is a Web Writer for Polymer Chemistry. She is currently a Global Marie Curie Fellow working alongside Professor Craig Hawker at the University of California, Santa Barbara (UCSB). Please visit this link for more information.