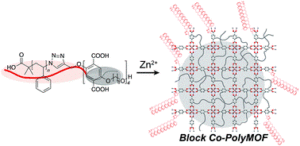
Block copolymer (BCP) assemblies are derived from covalently linked polymer chains and can undergo phase separation and thus find use in a wide range of applications including micropatterning, battery and electronic technologies. Metal-organic frameworks (MOFs) are another class of self-assembled matter consisting of crystalline networks with angstrom-scale order and permanent porosity. Owing to these advantageous properties, they can enable functions such as gas, energy storage, catalysis and selective-separation.
In the Johnson group, impressive efforts have been made to merge amorphous polymer networks with multi-component supramolecular assembly to generate soft materials with novel properties. In their current contribution, the group expands these types of hybrid materials by reporting a novel BCP where one block is a uniform benzene dicarboxylate (BDC)-based oligomer synthesized by iterative exponential growth (IEG), and the other is polystyrene (PS) prepared by atom transfer radical polymerization (ATRP). In order to achieve this, the authors initially synthesized the BDC-based oligomer with a defined end-functionality bearing an alkyne group that would allow for further diversification. This alkyne group was subsequently used to couple to azide-terminated polystyrene. In the presence of Zn ions, this BCP forms a “block co-polyMOF” (BCPMOF) material comprised of polyMOF domains embedded in a PS matrix.
The presented work is the first demonstration that it is possible to generate a crystalline polyMOF-amorphous polymer hybrid material from a single diblock copolymer. As such, BCPMOFs represent a new composite material that possesses the processability of the polymers while exhibiting enhanced stability towards ambient conditions when compared to the isolated MOFs. The ultimate goal of the group is to obtain BCPMOFs with robust mechanical properties, high surface areas, and tunable, well-defined domain sizes.
Tips/comments directly from the authors:
- In the synthesis of the mono benzylated diethyl 2,5-dihydroxyterephthalate, A, UV absorbance can readily distinguish the starting material from the product. The starting material elutes before the product and can be isolated for reuse.
- As noted in the supporting information, side products can occur during the coupling reaction to form L1. It is critical that ethanol is used to maintain the ethyl ester.
- The coupling reactions tend to require longer reaction times as the molecular weight of the reactants grows. Upon scaling up of the reactions, do not be temped to increase the concentration too much as it can lead to side product formation.
- The same synthetic protocols were used to form polyMOFs and block co-polyMOFs: 2.5 eq Zn(II) per BDC unit in Ln, was combined with Ln(PS) in DMF, heated at 100°C for 24h, followed by DMF washings to remove excess Zn(II) and unreacted ligand. Unlike the purification and isolation of L2 and L4, special care was taken for L4PS-Zn to use minimal organic solvent due to the solubility imparted by polystyrene.
Block co-polyMOFs: assembly of polymer-polyMOF hybrids via iterative exponential growth and “click” chemistry
Polym. Chem., 2017, 8, 4488-4493, DOI: 10.1039/c7py00922d
About the web writer
Dr. Athina Anastasaki is a web writer for Polymer Chemistry. She is currently a Global Marie Curie Fellow working alongside Professor Craig Hawker at the University of California, Santa Barbara (UCSB). Please visit this website for more information.