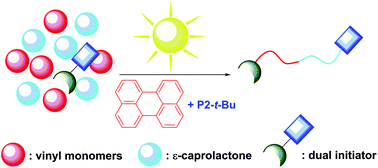
Block copolymers are of a great interest to the polymer chemistry community as they provide intermediate physicochemical properties when compared to the respective homopolymers. Sequential monomer addition, mechanistic transformation and coupling of different segments are three of the most popular approaches to obtain block copolymers. However, all these approaches suffer from several drawbacks such as being limited to monomers that can be polymerized only under the same polymerization mechanism or requiring extreme experimental precautions and elongated purification steps.
In order to address the latter challenge, Yagci, Yilmaz and co-workers developed the first metal free example of block copolymer formation in which they concurrently polymerize structurally different monomers from a junction point serving as two functional groups for each polymerization. Since atom transfer radical polymerization (ATRP) and ring opening polymerization (ROP) are not expected to interfere with each other and as such could be the ideal candidates for such a system. In order to test this hypothesis, a specifically designed initiator was synthesized possessing a tertiary bromine at one end (capable of initiating an ATRP reaction) and a hydroxyl functionality at the other end (capable for initiating a ROP reaction). Under carefully optimized conditions and in the presence of both the ATRP and ROP catalysts both methyl methacrylate (MMA) and ε- caprolactone could be simultaneously polymerized yielding a diblock copolymer with good agreement between theoretical and experimental value sand low dispersity values. Interestingly, the reaction took place on the roof of the chemistry department at Istanbul technical university utilizing natural sunlight as the light source. The applicability of this technique was further demonstrated by the simultaneous polymerization of different sets of monomers including n butyl acrylate- ε- caprolactone and methyl methacrylate-lactide combinations. As such, the successful combination of ATRP with ROP in the same reaction media allows for the facile one pot synthesis of block copolymers which can find use in further applications where excess of metals or inorganic residues would be undesirable.
Tips/comments directly from the authors:
1. All chemicals should be added into the reaction tube under nitrogen atmosphere in dark (perhaps by covering the outside of the tube with an aluminium foil) to avoid any premature light induced polymerization.
2. ROP polymerization can take place even in dark. Therefore, the ROP catalyst should be added last, and afterwards, the reaction tube should be exposed to sunlight as soon as possible. This way, one can provide the optimum conditions for the polymerizations to be realized simultaneously.
3. The method is best applicable in sunny days. Sunlight was deliberately selected as the most natural and simple way of light exposure. However, various other irradiation sources that emit in the wavelength regions matching with the absorption of appropriate sensitizers can also be used.
Read this exciting research for free until 17/07/2017 through a registered RSC account.
Block copolymer synthesis in one shot: concurrent metal-free ATRP and ROP processes under sunlight
Polym. Chem., 2017, 8, 2899-2903, DOI: 10.1039/c7py00069c
—————-
Dr. Athina Anastasaki is a web writer for Polymer Chemistry. She is currently a Global Marie Curie Fellow working alongside Professor Craig Hawker at the University of California, Santa Barbara (UCSB). Please visit this website for more