This month the focus is on Polymers and Rotaxanes, inspired by a recent lecture I attended, given by Sir J. Fraser Stoddart. He received the Nobel Prize in Chemistry in 2016, which was awarded jointly between himself, Jean-Pierre Sauvage and Bernard L. Feringa. His talk, which was part of the Krebs series of lectures at the University of Sheffield, featured his research in the field of mechanically interlocked molecules: rotaxanes and catenanes, which led to his pioneering work on “Molecular Machines”, for which the Nobel Prize was awarded.
Rotaxanes and catenanes are mechanically interlocked molecules; rotaxanes consist of a dumbbell shaped unit, with an encircled ring trapped by the dumbbell ends, whilst catenanes contain a number of interlocked macrocycles. The pioneering work of the Nobel laureates mentioned above has seen these systems developed, whereby through controlling the complexity and chemical functionality of the interlocked structures, work is possible, therefore leading to the term “Molecular Machines”.
Whilst rotaxane and catenane chemistry is well established, the same concept applied in polymer chemistry is currently a smaller area of research interest. However, this area of polymer chemistry research is emerging, with one article featured in Polymer Chemistry this month on the use of a non-polymeric rotaxane crosslinker to form toughened polymer networks. Looking back at previous Polymer Chemistry issues, two further articles utilising rotaxanes have been highlighted below.
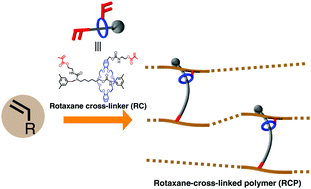
1. A vinylic rotaxane cross-linker for toughened network polymers from the radical polymerization of vinyl monomers
J. Sawada, D. Aoki, M. Kuzume, K. Nakazono, H. Otsuka, T. Takata
Polym. Chem., 2017, 8, 1878-1881; DOI: 10.1039/c7py00193b
The authors describe the use a non-polymeric rotaxane crosslinker (RC), with two polymerisable vinyl groups, one on each constituent of the rotaxane unit, for the preparation of rotaxane crosslinked polymers (RCP). The RCPS were prepared via the free radical polymerisation of either butyl acrylate or ethylhexyl acrylate with the RC. The RCPs showed a higher toughness and fracture energy when compared with an equivalent polymer crosslinked with a standard divinyl monomer.
2. Mechanically linked poly[2]rotaxanes constructed from the benzo-21-crown-7/secondary ammonium salt recognition motif
Peng Wang, Zhao Gao, Ming Yuan, Junlong Zhua, Feng Wang
Polym. Chem., 2016, 7, 3664-3668; DOI: 10.1039/c6py00494f
Here, poly[2]rotaxanes were prepared through Cu(I) catalysed click polymerisation of the individual rotaxane units to give a linear polymer. The rotaxane monomer was prepared via a so-called “threading-followed-by-stoppering” strategy. The rheological properties of the poly[2]rotaxanes were investigated and exhibited shear-thinning behaviour not observed for the poly(caprolactone) precursors.
3. Phototriggered supramolecular polymerization of a [c2]daisy chain rotaxane
Xin Fu, Rui-Rui Gu, Qi Zhang, Si-Jia Rao, Xiu-Li Zheng, Da-Hui Qu, He Tian
Polym. Chem., 2016, 7, 2166-2170; DOI: 10.1039/c6py00309e
The formation of a polyrotaxane was achieved through the initial protection of two 2-ureido-4-pyrimidinone (Upy) end groups with photolabile coumarin groups. Exposure to UV light removed the coumarin protecting groups, which led to the self-assembly of the Upy groups and supramolecular polymerisation of the [c2] daisy chain rotaxane monomer. This work represents an interesting stimuli-responsive supramolecular polymers utilising rotaxanes.
Read these articles for free until May 21th
Dr. Fiona Hatton is a web writer for Polymer Chemistry. She is currently a postdoctoral researcher in the Armes group at the University of Sheffield, UK. Find her on Twitter: @fi_hat