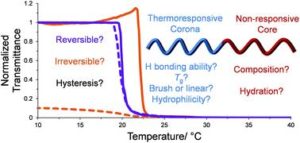
Blackman et al. report the synthesis of thermoresponsive polymers with tunable aggregation numbers in order to study the causes of thermal hysteresis.
Thermoresponsive polymers are materials that exhibit a change in their solubility over a temperature range. Thanks to this unique characteristic, these polymers can be used as smart and switchable materials for a wide range of biomedical applications. However, the cloud points upon cooling and heating a thermoresponsive polymer do not coincide because the process of equilibration takes time. The temperature interval between the cloud points upon cooling and heating is called hysteresis and the reversibility of these polymer’s thermal transitions can be influenced by many factors. In order to shine a light to these factors, O’Reilly, Gibson and Blackman synthesized well-defined, responsive amphiphilic block copolymers containing four different thermoresponsive corona blocks and assembled them in micellar structures in aqueous media. All micelles were designed to have tunable aggregation number enabling the study of the effects of altering the corona chemistry, chain confinement and core hydrophobicity on the thermoresponsive behavior, specifically the degree of hysteresis. It was found that higher core hydrophobicities were associated with a higher degree of hysteresis due to differences in core hydration. Linear corona chains capable of forming polymer-polymer hydrogen bonding interactions (e.g. pNIPAM) showed a greater hysteresis than those that could not (pDEAm). Importantly, the authors demonstrates that polymers with a brush-like architecture (pDEGMA and pOEGMA) exhibit irreversible phase transitions at a critical chain density, owing to irreversible nanoscale rearrangement in the precipitated bulk. These findings offer a deeper and more comprehensive understanding of stimuli-responsive self-assemblies and further highlight the complexity of hysteresis in thermoresponsive polymer systems.
Tips/comments directly from the authors:
1. It is important to use a Peltier system fitted with a reference cell with an internal temperature probe in order to accurately measure the hysteresis in the cooling curve. We have found that those without this feature typically over-estimate the hysteresis by a few degrees.
2. When synthesizing the pOEGMA-containing diblock copolymers, careful consideration of the RAFT CTA was necessary; the use of 2-Cyano-2-propyl dodecyl trithiocarbonate, as was employed for pDEGMA-containing diblock copolymers, resulted in a significant amount of high molecular weight polymers in the molecular weight distribution. Additionally, lower conversions had to be employed in order to reduce the presence of such species.
3. It was also important to employ multiple angle light scattering coupled with an algorithm such as REPES, which could enable us to investigate the molecular weights of the major fast mode and filter out scattering from spurious slow modes typically found in thermoresponsive polymer systems attributed to non-Brownian interactive behavior.
Read this paper for free until March 14th
Probing the causes of thermal hysteresis using tunable Nagg micelles with linear and brush-like thermoresponsive coronas
Polym. Chem., 2017, 8, 233-244.
DOI: 10.1039/C6PY01191H
Dr. Athina Anastasaki is a Web Writer for Polymer Chemistry. She is currently a Global Marie Curie Fellow working alongside Professor Craig Hawker at the University of California, Santa Barbara (UCSB). Please, visit her website for more information.