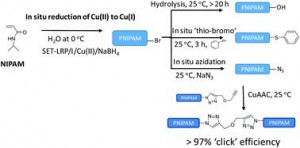
Gavrilov et al. report the quantitative and in situ functionalization of PNIPAM in aqueous media.
One major issue in aqueous copper mediated approaches is the hydrolysis of the halide end group which significantly compromises the end group fidelity of the resulting materials and therefore limits post polymerization modifications. In this current contribution, Monteiro and co-workers have carefully investigated the kinetics of hydrolysis of poly(N-isoproplyacrylamide) (PNIPAM) obtained via a new single electron transfer (SET) polymerization method to reduce Cu(II) directly and quantitatively to Cu(0). It was shown that the rate of hydrolysis is independent of the molecular weight of the polymer and the copper content in solution and reaches completion in approximately 15 hours.
In order to circumvent the hydrolysis issue, the authors elegantly conducted an in situ azidation of the bromine end group resulting in the quantitative transformation of the bromine end groups in a matter of 30 seconds, as indicated by matrix assisted laser desorption ionization time of flight mass spectrometry (MALDI-ToF-MS). This is quite a remarkable achievement given that organic solvents facilitate the same process in much longer reactions times, ranging from 10 to 24 hours. The fast reaction rate can be attributed to the enhanced solubility of sodium azide in water and the greater ion pair separation that facilitates nucleophilic attack.
In an attempt to further confirm the data obtained by MALDI-ToF-MS, the azide terminated PNIPAM was subsequently coupled to alkyne functional polymers (after purification) with coupling efficiencies greater than 97%. Thus, it was demonstrated that this approach can not only overcome the hydrolysis issues but also allow rapid synthesis of functional materials.
Tips/comments directly from the authors:
- The method of addition of the reactants is important, and by varying the order of addition the polymerizations rates can vary.
- It is important to note that the NaBH4 is hygroscopic, and care should be taken to store it in a water-free environment as errors can arise from weighting wet reagents.
- We believe that quantitative information can be obtained from the molecular weight distribution using the SEC/LND-model. This is especially important when determining quantitative coupling information of end-groups at high polymer molecular weights, as conventional techniques such as NMR and MALDI-ToF lose sensitivity. We use the weight distribution (i.e. w(M)) as the total weight of the reactants and products remains constant over the reaction; thus, the area under the w(M) vs M curve is the same before and after the reaction.
Read this exciting research for free until 30/09/2016 through a registered RSC account:
M. Gavrilov, Z. Jia,, V. Percec and M.J. Monteiro
Polym. Chem., 2016, 7, 4802-4809
DOI: 10.1039/C6PY00968A
—————-
Dr. Athina Anastasaki is a web writer for Polymer Chemistry. She is currently an Elings fellow working alongside Professor Craig Hawker at the University of California, Santa Barbara (UCSB).