Constantinou & Georgiou report the synthesis of thermoresponsive triblock copolymers using group transfer polymerisation.
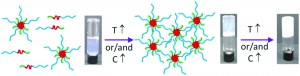
Thermoresponsive polymers can find use in a wide range of applications including tissue engineering and 3-D printing. For the successful synthesis of thermoresponsive gels several criteria need to be taken into account such as the composition, the molar mass, the concentration and the architecture. Georgiou’s group elegantly demonstrate the facile synthesis of such materials through group transfer polymerisation (GTP) thanks to its unique characteristics including scalability and faster reaction rates in comparison to conventional radical polymerisations. Different copolymers were targeted based on the ionic hydrophilic pH and thermoresponsive 2-(dimethylamino)ethyl methacrylate (DMAEMA), the non-ionic poly(ethylene glycol) (PEG)-based methacrylate (methoxy di-, penta-, and nona(ethylene glycol) methacrylate, DEGMA, PEGMA, and NEGMA), and the hydrophobic BuMA. The effect of the PEG side chain length and different compositions were systematically varied in order to investigate their effects on the thermoresponsive behaviour of the copolymers. Micelle formation was observed for all the terpolymers and the effective pKas were affected by the hydrophobic BuMA content and the architecture. Interestingly, the cloud points were affected by both the composition (BuMA content) and the PEG side group length and increase as the hydrophilic content and the PEG length increased. The gel points were investigated over a wide range of temperatures and concentrations and found to be influenced by both the composition and the PEG side chain length. Stable gels were formed by the most hydrophobic and with the shortest PEG length macromonomers. In summary, it was demonstrated that the sol–gel transition can be tailored by varying both the PEG length as well as the composition of the polymers.
Tips/comments directly from the authors:
- It is really important to monitor the temperature between additions during the one-pot synthesis. GTP is exothermic so when all monomer has converted to the polymer the temperature will drop back down so the next monomer can be added.
- Since each addition/polymerisation step takes about 10-15 the reaction can be monitored in real time by gel permeation chromatography, if necessary.
- Even though ideally all monomers have to be distilled this is not necessary when the GTP reaction is scaled up as long as the monomers are dry.
- Gelation is also influenced by ionic strength so if salt is added to the polymer solutions the solution will gel at lower temperatures and concentrations.
Thermoresponsive gels based on ABC triblock copolymers: effect of the length of the PEG side group by A. P. Constantinou and T. K. Georgiou , Polym. Chem., 2016, 7, 2045-2056
Dr. Athina Anastasaki is a Web Writer for Polymer Chemistry. She is currently an Elings fellow working alongside Professor Craig Hawker at the University of California, Santa Barbara (UCSB). Please visit her webpage for more information.










