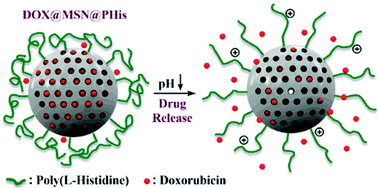
Bilalis et al. report the design and synthesis of novel pH-sensitive nanogates based on poly(L-histidine) from mesoporous silica nanoparticles.
The development of novel drug delivery materials necessitates the combination of the knowledge from different scientific fields, including organic and inorganic chemistry. Among the wide range of hybrid organic/inorganic materials, mesoporous silica nanoparticles (MSNs) have attracted considerable attention thanks to their unique characteristics such as high surface area, large specific volume, controllable pore diameter, facile surface functionalization and nontoxicity. On the other hand, polypeptide-coated silica-based systems, including poly(L-histidine) (PHis), have shown great promise in preventing untimely release of drugs and as such the combination of PHis and MSNs can provide an excellent template for the design of advanced drug delivery systems for controlled release applications. To this end, Iatrou, Bilalis and co-workers have exploited surface-initiated ring-opening polymerization (ROP) to synthesize novel pH-sensitive poly(L-histidine)-grafted mesoporous silica nanoparticles through an amino-functionalized MSN intermediate. The successful grafting of the homopolypeptide chains from the surface of MSNs was demonstrated by Fourier Transform-infrared spectroscopy (FT-IR), transmission electron microscopy (TEM) and thermogravimetric analysis (TGA) while size exclusion chromatography (SEC) confirmed the controlled character of the polymerization. Dynamic light scattering (DLS) and zeta potential analysis were also employed to ascertain the pH-responsive nature of the polypeptide-gated MSNs. In addition, drug loading and release studies were performed in order to verify the role of the grafted PHis chains as pH-sensitive nanogates for the MSN pores utilizing the model anticancer drug doxorubicin (DOX). DOX was efficiently loaded within the nanochannels of the hybrid MSN@PHis (~90%) and the drug entrapment and release pattern were proved to be pH-dependent with exert stability at physiological pH. The combination of the positive characteristics of MSNs and poly(L-histidine) enables the described materials as promising drug nanocarriers with potential in vitro and in vivo applications.
Tips/comments directly from the authors:
- It is really important to strictly maintain the reported time of reaction during the synthesis of MSNs using TEOS. Longer or less time of reaction will lead to larger or smaller nanoparticles, respectively.
- It should be noted that the functionalization of the surface of MSNs with APTES was conducted before the removal of CTAB so as to avoid the grafting of PHis chains from the MSN nanopores.
- When following the reported functionalization procedure, a LiOH solution must be used in order to remove HCl traces from the amino groups of MSNs after the removal of CTAB.
- The loading procedure of DOX into the MSN nanopores should take place at acidic pH (3.0). In that way the maximum drug encapsulation is ensured, because the PHis nanogates are in an opened state (fully protonated and thus hydrophilic).
pH-Sensitive nanogates based on poly(L-histidine) for controlled drug release from mesoporous silica nanoparticles by P. Bilalis, L.-A. Tziveleka, S. Varlas and H. Iatrou, Polym. Chem., 2016, 7, 1475-1485, DOI: 10.1039/C5PY01841B
Dr. Athina Anastasaki is a Web Writer for Polymer Chemistry. She is currently an Elings fellow working alongside Professor Craig Hawker at the University of California, Santa Barbara (UCSB). Please, visit her webpage for more information.