Zhang et. al. report a significant photoenhancement effect on classical ATRP and ATRP derivatives by ambient laboratory light.
Since the introduction of atom transfer radical polymerisation (ATRP) by Matyjaszewski and Sawamoto, a large diversity of other copper adjuncts have attracted considerable interest including activator regenerated by electron transfer (ARGET)-ATRP, activator generated by electron transfer (AGET)-ATRP, initiators for continuous activator regeneration (ICAR)-ATRP and single electron transfer living radical polymerisation (SET-LRP). Recently photoinduced copper radical polymerization has also drawn significant attention as CuBr2, typically referred to as deactivating species, can be reduced in situ generating the active CuBr species in the presence of UV or visible light. In this contribution, Jordan and co-workers investigated on whether the typical laboratory light would have any considerable impact on standard ATRP reactions. Interestingly, the vast majority of the ATRP techniques (with the exception of ARGET-ATRP) demonstrated a remarkable photoenhancement effect by ambient light, originated from common fluorescent lamps. It was observed that when less copper complex is utilized for the polymerizations, a stronger influence of the ambient light in the monomer conversion is evident and this effect was significant even in the presence of additional reducing agents. As a general rule, it was concluded that the slower the polymerization is, the more pronounced the effect of the ambient laboratory light can be. As it was proved that it makes a difference if one is performing an ATRP reaction with the hoods on and off, the effect of the laboratory light on the polymerization can no longer be neglected and the authors encourage the report of the light conditions in typical ATRP experiments in order to ensure the reproducibility.
Tips/comments directly from the authors:
Comments:
In this study, we provide conclusive evidence that common laboratory light especially originating from fluorescence lamps has a significant impact on ATRP. This is most probably one main reason why reproducibility of ATRP reactions are not as it should be which impairs further development of controlled radical polymerization techniques. As shown, the impact of light is different for the different ATRP recipes and will also strongly vary with the:
1. Type of complex formed regarding the metal and the ligands;
2. Quantity/concentration of metal complex formed and surely;
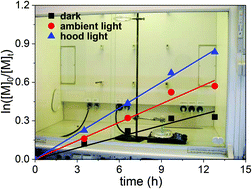
3. Type of illumination (natural light, type of fluorescent lamps installed in the laboratory and light intensity in the reaction vial). Strong influence were found for the “hood light illumination” (see Fig. 1 for description and Fig. 2 for experimental data) which was also very surprising for us. We therefore suggest to check the type of light bulbs/source of light, consider their emission spectra and resulting intensity at the location of the reaction and possibly provide these details in the experimental section to ensure reproducibility.
Tips:
1. The various metal complexes used in ATRP are most probably photosensitive but to a different extent. Thus, the influence of laboratory light will vary.
2. Perform the ATRP reactions always under the same light settings with the same light source and provide details (type of lamp, light intensity at each location of the reaction) in the experimental section.
3. Especially modern fluorescent lamps have a higher emission in the blue range and thus may have a stronger/other influence upon conversion/kinetics of the ATRP. Check the emission spectra of the lamps installed in the laboratory and especially in the chemical fume hoods.
Lights on! A significant photoenhancement effect on ATRP by ambient laboratory light by Tao Zhang Dan Gieseler and Rainer Jordan, Polym. Chem., 2016, 7, 775-779
Dr. Athina Anastasaki is a Web Writer for Polymer Chemistry. She is currently an Elings Fellow working alongside Professor Craig Hawker at the University of California, Santa Barbara (UCSB).Visit her webpage for more information.