This month, focussing on polymers in catalysis, we look at three articles where catalytic activity has been inferred to a polymer chain through functionalisation of the polymer, or through using the polymer as a support for another catalyst.
In the body, reactions are usually catalysed by enzymes. Mimicking enzyme activity with synthetic polymers has been investigated for several types of enzymes, here, in the first article a polymer was prepared mimicking the activity of S-adenosyl methionine synthetase.
Transition metal catalysis is widely used in the preparation of polymers as well as organic molecules, one major disadvantage is the removal of the catalyst after completion of the reaction. The second article describes a proposed solution to this problem through a thermoresponsive catalytic polymer. In the third article a porous polymer support containing in situ generated gold nanoparticles highlights another route to circumvent the issues with removal of catalytic residues, by utilising solid supported catalysts.
1. Synthetic polymeric variant of S-adenosyl methionine synthetase, Lakshmi Priya Datta, Binoy Maiti, Priyadarsi De, Polym. Chem., 2015, 6, 7796-7800.
The authors describe the synthesis of a polymer via RAFT and subsequent functionalisation with methionine moeities which mimicked the activity of the enzyme S-adenosyl methionine synthetase. Methionine plays major roles in the biosynthesis of proteins and DNA methylation. The resulting polymer was shown to methylate cytosine in the absence of a methyltransferase enzyme, highlighting the enzyme-like activity of the polymer.
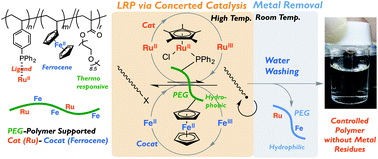
2. A thermoresponsive polymer supporter for concerted catalysis of ferrocene with a ruthenium catalyst in living radical polymerization: high activity and efficient removal of metal residues, Kojiro Fujimura, Makoto Ouchi, Mitsuo Sawamoto, Polym. Chem., 2015, 6, 7821-7826.
With the aim to achieve the efficient removal of metal residues from ruthenium-ferrocene concerted catalysed living radical polymerisation, a thermoresponsive polymer support was prepared containing ruthenium as a catalyst and ferrocene as a cocatalyst. This was used to catalyse the polymerisation of MMA in toluene, and subsequent aqueous washing resulted in the almost quantitative removal of Ru (99.8% removal) and Fe (98.5% removal), showing promise for practical applications.
3. “Clickable” thiol-functionalized nanoporous polymers: from their synthesis to further adsorption of gold nanoparticles and subsequent use as efficient catalytic supports, Benjamin Le Droumaguet, Romain Poupart, Daniel Grande, Polym. Chem., 2015, 6, 8105-8111.
A porous polymeric material was prepared through a channel die processing technique, consisting of PS-b-PLA, where the two blocks were connected through a disulphide linkage. After removal of the PLA block, the remaining thiol groups were utilised in both post-modification “click” reaction and in situ gold nanoparticle (GNP) generation. The porous polymer GNP hybrid catalysed the reduction of 4-nitrophenol to 4-aminophenol with a yield of 68%, and retained this efficiency over 5 runs.
Dr. Fiona Hatton is a Web Writer for Polymer Chemistry. She is currently a postdoctoral researcher at KTH Royal Institute of Technology, Sweden, having completed her PhD in the Rannard group at the University of Liverpool, UK. Visit her webpage for more information.