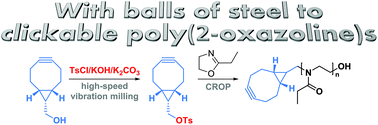
Glassner et. al. report a new solvent-free mechanochemical synthesis of a bicyclononyne tosylate.
The strain promoted azide-alkyne cycloaddition of cyclooctynes (SPAAC) is a popular bioorthogonal reaction that enables the tracking of biomacromolecules in living systems. Among the various cylooctynes that have been developed for SPAAC reactions, derivatives of bicyclo[6.1.0]non-4-yne (BCN) have attracted considerable interest for a number of reasons including their low hydrophobicity and ability to undergo fast cycloadditions not only with azides but also with nitrones. At the same time, poly(2-oxazoline)s (PAOx) represent a promising class of polymers for biomedical applications that can easily incorporate clickable end-groups by employing functional initiators or terminators. In this paper, Hoogenboom and co-workers present the first mechanochemical synthesis of a BCN tosylate (BCN-OTs) initiator via solvent-free reaction conditions utilizing high speed vibration milling instead of manual grinding. The BCN-OTs was subsequently used for the cationic ring opening polymerization (CROP) of 2-ethyl-2-oxazoline (EtOx) yielding a well-defined polymer and demonstrating controlled/living polymerization features such as narrow molecular weight distributions and high chain end fidelity. This represents a rapid route to prepare defined BCN functionalized PEtOx that can be used for bioorthogonal strain-promoted conjugation reactions. This was successfully demonstrated for the synthesis of a PS-b-PEtOx copolymer and a PEtOx-protein conjugate. As such, BCN-OTs may find potential applications as intermediate in the synthesis of functional BCN derivatives and BCN-PAOx. In addition, the ball-milling methodology utilized for this study is a promising tool for the synthesis of other unstable/highly reactive tosylates.
Tips/comments directly from the authors:
1) KOH and K2CO3 should be finely grounded and dried in a vacuum oven prior to use.
2) During the synthesis of BCN-OTs, removal of solvents has to be performed at ambient temperature (turn of the heating bath of the rotary evaporator).
3) BCN-OTs has to be used immediately for the next step because of its limited stability at ambient conditions.
Solvent-free mechanochemical synthesis of a bicyclononyne tosylate: a fast route towards bioorthogonal clickable poly(2-oxazoline)s by M. Glassner, S. Maji, V. de la Rosa, N. Vanparijs, K. Ryskulova, B. De Geest and R. Hoogenboom, Polym. Chem., 2015, 6, 8354-8359
Dr. Athina Anastasaki is a Web Writer for Polymer Chemistry. She is currently a Warwick (UK)/ Monash (Australia) research fellow working under the Monash Alliance. Visit http://haddleton.org/group-members for more information.