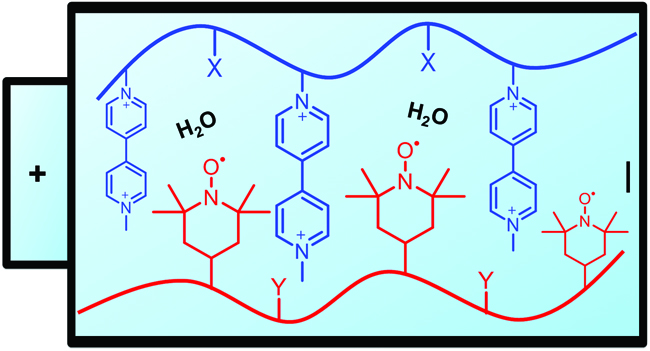
Janoschka et al. report TEMPO and viologen containing polymers for water-based redox-flow batteries.
Redox-flow batteries (RFBs) provide an inexpensive, safe and long lasting approach towards the development of stationary large-scale energy storage. Typically, RFBs employ redox- active materials that dissolve in either aqueous or organic solutions, although many of the existing systems challenge the chemical stability of installed battery components and impose a safety risk. Vanadium salts in sulphuric acid consist the most thoroughly studied system and when aqueous solutions are additionally employed further advantages can be achieved including low corrosivity, high safety and affordable size-exclusion membranes. In this current contribution, Schubert and co-workers present the screening of two series of redox-active polymers that can potentially find application in water-based polymer redox-flow batteries (pRFBs). The rheological and electrochemical properties of both viologen and TEMPO containing polymers have been investigated in details. The viologen-homopolymer poly(1-methyl-1’-(4-vinylbenzyl)-[4,4’-bipyridine]-1,1’-diium dichloride) was found to exhibit good solubility in water (even in the reduced state) and a redox potential of -0.4 V (vs Ag/AgCl), demonstrating its suitability as an anode material. On the other hand, the TEMPO-polymer showed reduced solubility in aqueous solution and thus requiring a solubilizing comonomer. Although poly(ethyleneglycol)-based comonomers and methacrylamide were subsequently tested, the unfavourable LCST and the requirement of high mole fractions respectively led to the investigating of a third alternative. Pleasingly, poly(4-methacryloyloxy-2,2,6,6-tetramethylpiperidine-1-oxyl-co-2-(methacryloyloxy)-N,N,N-trimethylethane ammonium chloride) proved to be a suitable cathode material for pRFBs when a choline moiety was used as the solubilizing unit. In summary, the presented work paves the way for the production of economical energy storage devices that are safe, metal free and utilise all-organic raw materials.
Tips/comments directly from the authors:
1. It should be noted that the redox-active polymer solutions can be used straight from the reaction vessel and without further purification for the designated application in pRFBs.
2. The TEMPO-polymers are excellent surfactants and prone to foam formation. The authors highly encourage the reader to come up with ideas for potential applications of this redox-active, radical-bearing surfactant.
3. When preparing polymers for pRFBs the molar mass of the polymer should be tailored in order to fit the size-exclusion membrane (dialysis membrane) used in the battery. The dispersity Đ should be low to ensure good rheological properties.
Synthesis and characterization of TEMPO- and viologen-polymers for water-based redox-flow batteries, by T. Janoschka, S. Morgenstern, H. Hiller, C. Friebe, K. Wolkersdörfer, B. Häupler, M.D. Hager and U.S. Schubert, Polym.Chem., 2015, 6, 7801-7811
Dr. Athina Anastasaki is a Web Writer for Polymer Chemistry. She is currently a Warwick (UK)/ Monash (Australia) research fellow working under the Monash Alliance. Visit http://haddleton.org/group-members for more information.