Prof. Jacques Lalevée was born in Remiremont (France) in 1976. After studying Physical Chemistry at the University of Nancy (now University of Lorraine), he received his Ph.D. degree in Materials Chemistry under the supervision of Prof. Jean Pierre Fouassier from Mulhouse in 2002. After approximately one-year of postdoctoral research with Prof. Gerd Kothe (Germany), he joined the “Ecole Nationale Supérieure de Chimie de Mulhouse (ENSCMu)” in September, 2004. He was promoted to full professor in September, 2009. Since 2011, Jacques has been a Professor at the “Institut Universitaire de France (IUF-Paris)”. His current research interests encompass free radical chemistry, the design of new (photo)polymerisation initiating systems and new polymers, as well as mechanistic elucidation in polymer chemistry. He has published nearly 200 peer-reviewed papers with an H-index of 29.
Prof. Jacques Lalevée was born in Remiremont (France) in 1976. After studying Physical Chemistry at the University of Nancy (now University of Lorraine), he received his Ph.D. degree in Materials Chemistry under the supervision of Prof. Jean Pierre Fouassier from Mulhouse in 2002. After approximately one-year of postdoctoral research with Prof. Gerd Kothe (Germany), he joined the “Ecole Nationale Supérieure de Chimie de Mulhouse (ENSCMu)” in September, 2004. He was promoted to full professor in September, 2009. Since 2011, Jacques has been a Professor at the “Institut Universitaire de France (IUF-Paris)”. His current research interests encompass free radical chemistry, the design of new (photo)polymerisation initiating systems and new polymers, as well as mechanistic elucidation in polymer chemistry. He has published nearly 200 peer-reviewed papers with an H-index of 29.
He was awarded the Guy Ourisson 2013 Prize as well as the national prize of the French polymer group (GFP) in 2014.
What was your inspiration in becoming a chemist?
I became really interested in chemistry at high school. I was fascinated by chemical reactions and the possibility of understanding events at this molecular scale. I have always been interested in free radical chemistry as many different reaction pathways can be expected from these chemical species. The “positive” use of free radicals in polymerisation processes was always a challenge for me; particularly the possibility of triggering processes by light for perfect time and spatial controls.
What was the motivation to write your Polymer Chemistry article?
Light-induced polymerisation technique is a promising approach for the fabrication of various polymeric materials due to its environmental, economic and production benefits. This technique is mainly based on the photochemically generated reactive species (e.g. radicals or cations, produced from the photochemical reactions of photoinitiating systems after the absorption of light) to rapidly transform the specially formulated reactive liquids to solids (3D polymeric networks for various materials) at room temperature. Recently, light-emitting diodes (LEDs) have attracted increased attention as potential irradiation sources for photopolymerisation processes substituting traditional mercury UV lamps; their advantages include being more environmentally friendly, having better light output, higher operating efficiency and lower cost and energy consumption.
Recently, the use of metal based complexes as photoredox catalysts in polymer science has generated lots of interest and is actually a huge challenge. In the present paper, we propose a new iridium complex (Ir(btp)2(tmd)) as a novel photoredox catalyst with enhanced efficiency under visible lights (laser diodes, LEDs and household halogen lamp) for i) cationic polymerisation, ii) free radical polymerisation, iii) controlled/living radical polymerisation and iv) polymer surface modification, including micropatterning by laser direct writing.
Why did you choose Polymer Chemistry to publish your work?
The Royal Society of Chemistry is clearly one of the leading societies and accordingly its polymer journal “Polymer Chemistry” has rapidly emerged as a leader journal in Polymer Science. The wide international readership, the quick submission and review system are also particularly interesting.
In which upcoming conferences may our readers meet you?
The European Polymer Congress (Dresden, Germany in June 2015) or 11th International Symposium on Ionic Polymerization (Bordeaux – France July 2015).
How do you spend your spare time?
Obviously, I am willing to spend more time with my family. I like cycling, and would like to dedicate more time to it.
Which profession would you choose if you were not a scientist?
I would become a teacher in middle school, it was my original idea, but I met excellent professors in the University and they have opened my mind to research.
Sofia Telitel, Frederic Dumur, Siham Telitel, Olivier Soppera, Marc Lepeltier, Yohann Guillaneuf, Julien Poly, Fabrice Morlet-Savary, Philippe Fioux, Jean-Pierre Fouassier, Didier Gigmes and Jacques Lalevée
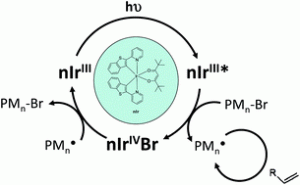
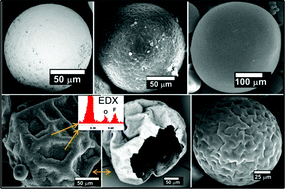
A new iridium complex (nIr) was designed and investigated as a photoinitiator catalyst for radical and cationic polymerization upon very soft irradiations (lights ranging from 457 to 532 nm). A ring-opening polymerization (ROP) of an epoxy monomer was easily promoted through the interaction between nIr and an iodonium salt (Iod) upon light. In radical polymerization, nIr can be efficient in combination with phenacyl bromide (PBr) and optionally an amine. These photoinitiating systems work according to an original oxidative cycle and a regeneration of nIr is observed. A control of the methyl methacrylate polymerization (conducted under a 462 nm light) with 1.2–1.6 polydispersity indexes was displayed. Surface modifications by direct laser write was also easily carried out for the first time through surface re-initiation experiments, i.e. the dormant species being reactivated by light in the presence of nIr; the polymer surfaces were analyzed by XPS. The chemical mechanisms were examined through laser flash photolysis, NMR, ESR and size exclusion chromatography experiments.
Cyrille Boyer is a guest web-writer for Polymer Chemistry. He is currently an Associate Professor and an ARC-Future Fellow in the School of Chemical Engineering, University of New South Wales (Australia).












 Prof. Jacques Lalevée was born in Remiremont (France) in 1976. After studying Physical Chemistry at the University of Nancy (now University of Lorraine), he received his Ph.D. degree in Materials Chemistry under the supervision of Prof. Jean Pierre Fouassier from Mulhouse in 2002. After approximately one-year of postdoctoral research with Prof. Gerd Kothe (Germany), he joined the “Ecole Nationale Supérieure de Chimie de Mulhouse (ENSCMu)” in September, 2004. He was promoted to full professor in September, 2009. Since 2011, Jacques has been a Professor at the “Institut Universitaire de France (IUF-Paris)”. His current research interests encompass free radical chemistry, the design of new (photo)polymerisation initiating systems and new polymers, as well as mechanistic elucidation in polymer chemistry. He has published nearly 200 peer-reviewed papers with an H-index of 29.
Prof. Jacques Lalevée was born in Remiremont (France) in 1976. After studying Physical Chemistry at the University of Nancy (now University of Lorraine), he received his Ph.D. degree in Materials Chemistry under the supervision of Prof. Jean Pierre Fouassier from Mulhouse in 2002. After approximately one-year of postdoctoral research with Prof. Gerd Kothe (Germany), he joined the “Ecole Nationale Supérieure de Chimie de Mulhouse (ENSCMu)” in September, 2004. He was promoted to full professor in September, 2009. Since 2011, Jacques has been a Professor at the “Institut Universitaire de France (IUF-Paris)”. His current research interests encompass free radical chemistry, the design of new (photo)polymerisation initiating systems and new polymers, as well as mechanistic elucidation in polymer chemistry. He has published nearly 200 peer-reviewed papers with an H-index of 29.