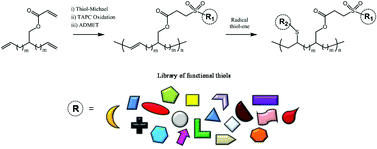
The preparation of functional α,ω-dienes via thiol-Michael chemistry including synthesis, oxidative protection, acyclic diene metathesis (ADMET) polymerization and radical thiol–ene modification has been reported by van Hensbergen et al.
The synthesis of the novel α,ω-diene 2-(undec-10-en-1-yl)tridec-12-en-1-yl acrylate is described. Thiol-Michael coupling of this substrate followed by chemoselective oxidation of the thioether moiety with triazotriphosphorine tetrachloride (TAPC) furnished a suite of functional and symmetrical ADMET-active monomers in a quick and convenient manner. Polymerization of these adducts with Grubbs 1st generation catalyst (RuCl2(PCy3)2CHPh) was demonstrated to high conversion, and quantitative radical initiated thiol–ene modification of the backbone C![[double bond, length as m-dash]](http://www.rsc.org/images/entities/char_e001.gif) C bonds was performed to impart additional functionality to each ADMET polymer. These reactions highlight the compatibility of thiol-based click chemistries for the preparation and post-modification of functional ADMET materials.
C bonds was performed to impart additional functionality to each ADMET polymer. These reactions highlight the compatibility of thiol-based click chemistries for the preparation and post-modification of functional ADMET materials.
Functional α,ω-dienes via thiol-Michael chemistry: synthesis, oxidative protection, acyclic diene metathesis (ADMET) polymerization and radical thiol–ene modification by Johannes A. van Hensbergen, Taylor W. Gaines, Kenneth B. Wagener, Robert P. Burford and Andrew B. Lowe Polym. Chem., 2014, 5, 6225-6235.
Remzi Becer is a web-writer and advisory board member for Polymer Chemistry. He is currently a Senior Lecturer in Materials Science and the director of the Polymer Science and Nanotechnology masters programme at Queen Mary, University of London. Visit www.becergroup.com for more information.