Monomers based on vinylcyclopropane (VCP) have not attracted a lot of attention in polymer chemistry compared to regular vinyl monomers. This is mainly due to the difficulty in synthesizing them and their polymerisation behaviour. Polymerisation of VCP is known to proceed via a 1,2-type or a 1,5-type, i.e. a radical ring opening polymerization (RROP). RROP of VCP can lead to three different isomeric repeating units: two pent-2-enyl units and one cyclobutyl unit. These different polymerization pathways are the reason why VCP derivatives are not frequently used in polymer chemistry.
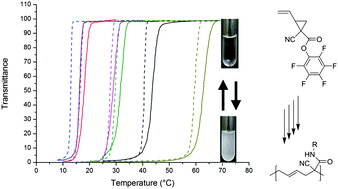
Theato et al. have demonstrated the synthesis and polymerization of a new reactive vinylcyclopropane monomer, 1-cyano-1-pentafluorophenoxycarbonyl-2-vinylcyclopropane. The obtained polymer contained exclusively the pent-3-enyl repeating unit in the polymer backbone. Taking advantage of activated ester chemistries, post-polymerization modifications with different aliphatic amines have been conducted. All prepared polymers exhibited an upper critical solution temperature (UCST) in ethanol and ethanol–water. It was found that the UCST was directly related to the amide moiety of the polymer. An increased solubility of the polymer in ethanol with the increase of the volume of the aliphatic amide moiety was observed. The reverse effect was found when adding water to the ethanolic solution of the polymer.
Post-polymerization modification of reactive polymers derived from vinylcyclopropane: 1. synthesis and thermo-responsive behaviour by Denis H. Seuyep N., Gerrit A. Luinstra and Patrick Theato Polym. Chem., 2013, 4, 2724-2730.