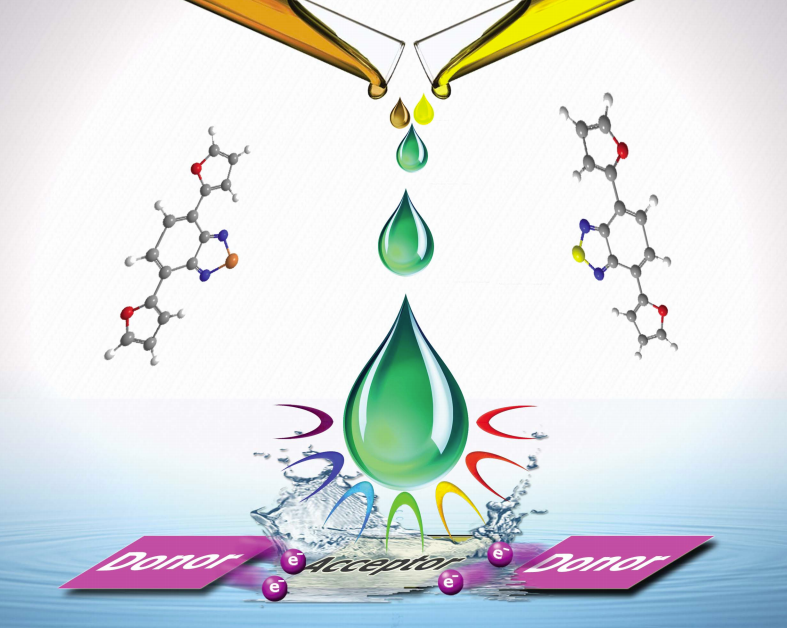
Conjugated aromatic polymers have attracted great attention since 1970s. Myriad of studies have been reported on five-membered heterocycles containing polymers such as polypyrroles, polythiophenes and their derivatives due to their susceptible electronic and optical properties. Recent studies showed that furan containing polymers have some priorities over thiophene based ones for the application of polymer organics in advanced technological applications. For instance, the greater electron withdrawing ability of furan is capable of reducing the HOMO energy level of the D parts in the solar cells, which results in the high open circuit voltage.
Cihaner, Onal and their coworkers have designed and synthesized two new furan and benzochalcogenodiazole based monomers, namely 4,7-di(furan-2-yl)benzo[c][1,2,5]-selenadiazole (FSeF) and 4,7-di(furan-2-yl)benzo[c][1,2,5]thiadiazole (FSF), via a donor–acceptor–donor approach. The monomers were electrochemically polymerized via potentiodynamic or potentiostatic methods. The monomers and their polymers exhibited lower oxidation potentials (1.16 V and 1.06 V for monomers; 0.93 V and 0.80 V for polymers vs. Ag/AgCl) and red shifts of the whole dual-band absorption spectra upon moving from S to Se. Intramolecular charge transfer properties of the monomers and the polymers were demonstrated by using electroanalytical and optical methods. Also, the polymers PFSeF and PFSF were multicolored at different redox states and have low band gaps of 1.43 eV and 1.61 eV, respectively.
Furan and benzochalcogenodiazole based multichromic polymers via a donor–acceptor approach by Merve İçli-Özkut, Halil İpek, Baris Karabay, Atilla Cihaner and Ahmet M. Önal Polym. Chem., 2013, 4, 2457-2463