To keep up-to-date with all the latest research, sign up for the journal’s e-alerts or RSS feeds or follow Polymer Chemistry on Twitter or Facebook.
Archive for November, 2012
Paper of the week: Single chain polymeric nanoparticles as compartmentalised sensors
27 Nov 2012
The unique physical properties of supramolecular polymers resulted in a myriad of potential applications ranging from electronics to healthcare and high performance materials. The large spectrum of available self-assembling molecules allows the properties of supramolecular polymers to be tuned to specific requirements of the desired applications. It has been recently shown that linear polymers grafted with non-covalent (or dynamic covalent) interacting groups lead, under selected conditions, to the folding of single polymeric chains into what are now termed single chain polymeric nanoparticles (SCPNs). Due to the unique properties of SCPNs, these well-defined nanometer-sized objects are actively investigated for use in advanced applications in low viscosity coatings, catalytic systems and nanomedicine.
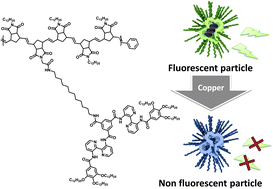
In this context, Palmans and co-workers envisionned chemosensing of metal ions as an interesting application of SCPNs. The authors prepared 3,3′-bis(acylamino)-2,2′-bipyridine substituted benzene-1,3,5-tricarboxamide (BiPy-BTA) grafted polynorbornene polymers. The polymers fold intramolecularly via p–p interactions into fluorescent, compartmentalised particles of nanometer-size. Spectroscopic and light scattering techniques show that the compact conformation of the folded polymer is affected by increasing the BiPy-BTA functionalisation degree and by changing the solvent polarity. Changes in the conformation are accompanied by changes in the fluorescence intensity. Due to the affinity of the BiPy units for metal ions such as copper, the particles obtained are effective sensors for these metals. The compartmentalisation of the binding motifs in SCPNs proves to be advantageous in sensor applications of these particles.
Single chain polymeric nanoparticles as compartmentalised sensors for metal ions by Martijn A. J. Gillissen, Ilja K. Voets, E. W. Meijer and Anja. R. A. Palmans, Polym. Chem., 2012, 3, 3166-3174.
Paper of the week: Role of multitopicity in hydrogen bonded supramolecular polymers
13 Nov 2012
Hydrogen bonding interactions in aqueous media are often very weak because of the competition from water molecules, but they can still have a decisive effect on self-assemblies when used in combination with other interactions. In the case of amphiphiles with a hydrophobic part made from flexible alkyl chains, the introduction of hydrogen bonds within the hydrophobic domains through urea groups has been shown to dramatically increase the viscosity of aqueous solutions, and to enable self-sorting between amphiphiles of distinct structures. Another popular approach to synthesise viscous solutions or gels consists in decorating water soluble high molar mass polymers with hydrophobic groups.
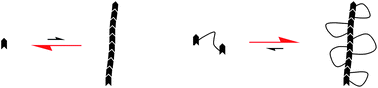
In this article, Bouteiller and co-workers investigated the properties of systems combining both design elements, i.e. macromolecules with hydrophobic groups able to form very long anisotropic hydrophobic domains. A strong influence of both the number of associative groups per chain and the polydispersity has been demonstrated. In water, where the interactions between stickers are strong, the monomer self-assembles into filaments, but all other compounds with more than one sticker per chain are insoluble. In methanol, where the interactions between stickers are weaker, neither the monomer nor the monodispersed dimer is assembled, whereas polydispersed chains with an average number of stickers per chain of 2 or 3 self-assemble into filaments, leading to macroscopic gelation.
Hydrogen bonded supramolecular polymers in protic solvents: role of multitopicity by Marion Tharcis, Thomas Breiner, Joël Belleney, François Boué and Laurent Bouteiller, Polym. Chem., 2012, 3, 3093-3099.
To keep up-to-date with all the latest research, sign up for the journal’s e-alerts or RSS feeds or follow Polymer Chemistry on Twitter or Facebook.
Paper of the week: Fluorescence resonance energy transfer in recognition-mediated polymer-quantum dot assemblies
05 Nov 2012
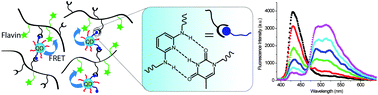
Organization of nanoparticles (NPs) into morphologically controlled and organised structures is a central issue for bottom-up fabrication of functional devices in optoelectronics, sensing, catalysis and medicine. Directed host–guest assembly of NPs into polymer matrices is an effective route to form structured NP assemblies with advantageous optical, electronic, magnetic, and mechanical properties. In this article, Cooke, Rotello and co-workers reported recognition mediated assembly of ZnSe quantum dots (QDs) with a chromophore-functionalized polymer, facilitating fluorescence resonance energy transfer (FRET) from QDs to the chromophore. The authors designed and synthesized a polyfunctional copolymer featuring a solubilising methyl methacrylate (MMA) element, a diamidopyridine (DAP) recognition element and a flavin (Fl) chromophore. Thymine functionalized ZnSe QDs (Thy-QDs) were used as the guest in the assembly. Due to the spectral overlap and close proximity of the QDs and flavin units in the assembly, FRET was observed from QDs to flavin. This methodology of producing self-assembled structures both in solution and solid state provides a powerful tool for the creation of highly structured multifunctional materials and devices.
Fluorescence resonance energy transfer in recognition-mediated polymer-quantum dot assemblies by Vikas Nandwana, Brian Fitzpatrick, Qian Liu, Kyril M. Solntsev, Xi Yu, Gülen Yesilbag Tonga, Serkan Eymur, Murat Tonga, Graeme Cooke and Vincent M. Rotello, Polym. Chem. 2012, 3, 3072-3076.
To keep up-to-date with all the latest research, sign up for the journal’s e-alerts or RSS feeds or follow Polymer Chemistry on Twitter or Facebook.