In the search for new haircare products, scientists in the UK have developed a new method to chemically modify hair with polymers.
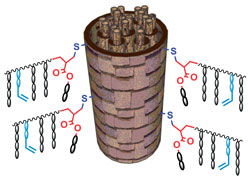
The polymer can be covalently grafted to the surface of hair under mild conditions
Polymers are present within many haircare products and are used to modify the appearance of hair, for example to make it straighter or to change its colour. These polymers modify the surface of the hair and as they are used in a personal and uncontrolled setting, mild and efficient chemistry is required. Typically, there is a non-covalent interaction between the polymer and hair; however, scientists are now looking at methods to form a covalent bond between the polymer and hair in the hope of enhancing the polymer’s effects.
David Haddleton and coworkers at the University of Warwick, in collaboration with Unilever, have demonstrated for the first time the ability to covalently bond polymers synthesised by cobalt catalysed chain transfer polymerisation (CCTP) to hair.
CCTP is a method to synthesise macromonomers that have a reactive unsaturated vinyl end-group, which can react with thiol groups found on the surface of hair using thiol-ene click chemistry under mild conditions. In addition, the team also showed that a fluorescent tag could be attached to the polymer, demonstrating the further modification of the polymer. ‘The use of low-cost controlled polymerisation methods to give efficient and specific surface modification onto surfaces is highly exciting,’ says Ezat Khoshdel from Unilever.
Now that it is known that polymers can be covalently bound to hair, the team is ‘interested in what type of properties we can change, and what are essentially the limitations of what we are doing,’ says Haddleton.
Greg Qiao, an expert in polymer chemistry at the University of Melbourne, Australia, says that ‘the work provides a general method for modification of a biological surface and has the potential to produce new healthcare products for human hair’.
Biological surface modification by ‘thiol-ene’ addition of polymers synthesised by catalytic chain transfer polymerisation (CCTP)
Stacy Slavin, Ezat Khoshdel and David M. Haddleton
DOI: 10.1039/C2PY20040F
Read the original Chemistry World article here










