Imagine a scenario where one morning, a close friend of yours calls you and painfully conveys the news of him being diagnosed with cancer and you, instead of sitting horrified and helpless, casually say “Hey don’t worry man, we have PDT!” That sounds fascinating right?! Yes, Photodynamic therapy has shown potential to do that. With the same fascination towards the idea of photodynamic therapy, inventors of PDT pursued research on this therapy and shaped an unconventional out of box method of treating cancer. The simple mechanism of working of this technique is widely known. Drugs used in this technique are light sensitive. In response to specific light irradiated on the drug molecule, it converts surrounding molecular oxygen into form of oxygen which kills nearby cancer cells. The reasons this therapy called as out of box here are multifold. First, there are many photosensitizers easily available approved by FDA which can easily respond to specific light and produced the effect explained above. Second it makes use of naturally available oxygen molecules surrounding cancer cells. Last and importantly all the conventional drugs/ therapies for the cancer are immunosuppressive meaning they suppress our immune system after treatment unlike PDT, which is immunostimulative which stimulates immune system of the patient after treatment.
Proving very promising in its early years of research, PDT faces a major challenge. Light sensitive drugs usually respond to visible light. Visible light’s penetration in the tissues and cells is poor because it is mostly absorbed and hence the drug does not get sufficient light to tackle cancer cells (This is the low penetration depth of visible light). This is a major bottleneck in PDT’s usage and is being addressed by researchers all over the globe.
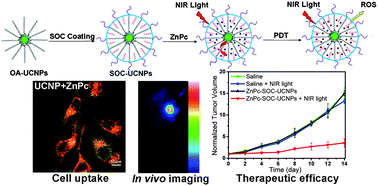
In one very fine effort by Sisi Cui etal they try to address the problem stated above in very interesting way. The key fact they have exploited here is though visible light’s penetration in the tissue and biological matter is weak but near infer red (NIR) light can penetrate deeply in the same tissue without any problem. So NIR light can be good alternative to visible light. Here Sisi Cui etal synthesized NaYF4(Er,Yb) nanoparticles ( upconversion nanoparticles) which can convert NIR light to visible light. A chitosan derivative (biocompatible polymer) has coated these nanoparticles with drugs deposited on their surface. So upconversion nanoparticles and light sensitive drug are in close proximity of each other. So when NIR light is irradiated, upcoversion nanoparticles convert that light in visible light( drug is essentially insensitive to NIR light) and transfers that light to the drug which in response coverts surrounding oxygen from the molecular form to the form which can kill the cancer cells. This study which is cited below has not only addressed the problems associated with the synthesis of hydrophilic NaYF4 nanoparticles brilliantly, but also shown that the scheme they have proposed works successfully which they have demonstrated actually by killing in vitro cancer cells.
Amphiphilic chitosan modified upconversion nanoparticles for in vivo photodynamic therapy induced by near-infrared light
Sisi Cui, Haiyan Chen, Hongyan Zhu, Junmei Tian, Xuemei Chi, Zhiya Qian, Samuel Achilefu and Yueqing Gu
J. Mater. Chem. C, 2012, 22, 4861-4873. DOI:10.1039/C2JM16112E
Padmanabh Joshi is a guest web writer for the Journal of Materials Chemistry blog. He currently works at the Department of Chemistry, University of Cincinnati.
To keep up-to-date with all the latest research, sign-up to our RSS feed or Table of contents alert.