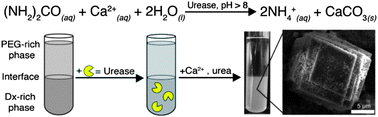
Although the chemistry of life is usually considered to be organic, living organisms also need to carry out inorganic synthesis. The formation of bones, teeth and shells all depend on the ability of cellular machinery to efficiently prepare inorganic materials with the correct morphology. This machinery is complex and involves the concerted action of small molecules, enzymes and high concentrations of macromolecules.
It is the role of the macromolecules that is the focus of a recent paper by David N. Carace and Christine D. Keating. They aimed to mimic the formation of CaCO3 by conducting a mineralization reaction in an aqueous two-phase system (ATPS). This system consisted of two immiscible polymer solutions: a poly(ethylene glycol) (PEG) solution floating on a solution of dextran. The mineralisation reaction involved the urease–mediated conversion of urea to carbonate ions and their subsequent reaction with calcium. It was found that biomineralization occurred predominantly in the dextran layer due to the localization of urease. It was also found that decreasing the relative volume of the dextran phase increased the rate of CaCO3 formation.
This work has demonstrated a fascinating method of biological reaction compartmentalization that does not rely on membranes.
Biocatalysed mineralization in an aqueous two-phase system: effect of background polymers and enzyme partitioning
David N. Cacace and Christine D. Keating
J. Mater. Chem. B, 2013, 1, 1794. DOI:10.1039/C3TB00550j
James Serginson is a guest web writer for the Journal of Materials Chemistry blog. He currently works at Imperial College London carrying out research into nanocomposites.
To keep up-to-date with all the latest research, sign-up to our RSS feed or Table of contents alert.