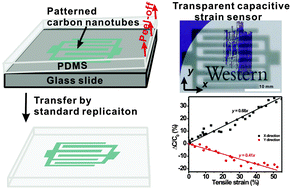
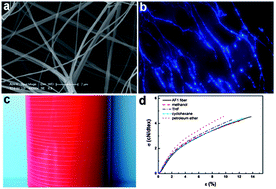
One of the most promising areas of materials chemistry research is the creation of synthetic analogues of biological tissue. The development of such materials would reduce the need for transplants and their associated problems. For example, polymer-based, artificial vascular grafts are a promising candidate for use in bypass operations. Unfortunately, they increase the risk of increasing thrombus (blood clot) formation. This in turn can lead to risk of serious medical problems such as stroke, heart attack and pulmonary embolism.
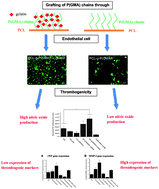
Researchers at Nanyang Technological University in Singapore have found that using functionalised polycaprolactone (PCL) can lead to reduced thrombogenicity. PCL itself is already widely used for in vivo applications although its hydrophobicity reduces its usefulness as an artificial blood vessel. Hydrophobic materials are also thought to be unsuitable because of their weak interactions with endothelial cells (ECs) – something commonly implicated in thrombus formation. To overcome these limitations, poly(glycidyl methacrylate) (PGMA) was grown from PCL surfaces via a graft-from living polymerisation. The side chains of the PGMA were then used to attach gelatine molecules. It was found that the gelatin coat decreased the hydrophobicity of the surface leading to improved EC adhesion and a corresponding reduction in thrombogenicity.
Endothelial cell thrombogenicity is reduced by ATRP-mediated grafting of gelatin onto PCL surfaces
Gordon Minru Xiong, Shaojun Yuan, Chek Kun Tan, Jun Kit Wang, Yang Liu, Timothy Thatt Yang Tan, Nguan Soon Tan and Cleo Choong
J. Mater. Chem. B, 2014, 2, 485-493. DOI:10.1039/C3TB20760a
James Serginson is a guest web writer for the Journal of Materials Chemistry blog. He currently works at Imperial College London carrying out research into nanocomposites.
To keep up-to-date with all the latest research, sign-up to our RSS feed or Table of contents alert.