 Cinzia Chiappe is a Professor of Organic Chemistry at the University of Pisa, Italy. Her research interests primarily focus on ionic liquids and their biological and physic chemical properties. The ultimate goal of her research is to design optimised ionic liquids as solvents and/or catalysts for sustainable chemical reactions. Cinzia took a few moments to chat to Green Chemistry…
Cinzia Chiappe is a Professor of Organic Chemistry at the University of Pisa, Italy. Her research interests primarily focus on ionic liquids and their biological and physic chemical properties. The ultimate goal of her research is to design optimised ionic liquids as solvents and/or catalysts for sustainable chemical reactions. Cinzia took a few moments to chat to Green Chemistry…
Who or what initially inspired you to become a chemist?
During my studies I was at first attracted by mathematics and biology and I thought I would become a “biologist”. Subsequently, in the last years of high school, my interest moved towards the single mechanisms that determine and govern the life on this planet. I discovered my interest for “molecules” and their interaction ability and so I decided to study chemistry. I therefore became an Organic Chemist.
What has been the motivation behind your recent research?
As an organic chemist, I studied reactivity and reaction mechanisms. At the beginning of this century (1999-2000), I discovered the fascinating world of ionic liquids and immediately I was attracted by these compounds for the copious challenges and potentialities that they offer to a researcher involved in “organic reactivity”. The subsequent step, from ionic liquids to “green chemistry”, was only a short step.
What do you see as the main challenges facing research in this area?
The main challenges are related to the possibility of resolving some strategic problems for this society, i.e. the depletion of our principal source of energy and organic compounds (fossil fuels) as well as the depletion of other important primary materials (some metals and metal salts).
Where do you see the field of green chemistry being in 5 or 10 years time?
I think that green chemistry and the application of its principles in different areas (energy, material sciences, waste disposal and so on) can become a strategic approach (probably, the only one) to overcome the problems characterizing this “small” planet with “many” inhabitants and “few” resources. Of course, small, many and few are strictly related quantities.
If you could not be a scientist, but could be anything else, what would you be?
I don’t know, but probably an “archistar” – a superstar architect.
Take a look at a few of Cinzia’s recent Green Chemistry articles below – all free to access:
A dramatic effect of the ionic liquid structure in esterification reactions in protic ionic media, Cinzia Chiappe, Sunita Rajamani and Felicia D’Andrea, Green Chem., 2013, 15, 137-143
Synthesis and properties of trialkyl(2,3-dihydroxypropyl)phosphonium salts, a new class of hydrophilic and hydrophobic glyceryl-functionalized ILs, Fabio Bellina, Cinzia Chiappe and Marco Lessi, Green Chem., 2012, 14, 148-155
Styrene oxidation by hydrogen peroxide in ionic liquids: the role of the solvent on the competition between two Pd-catalyzed processes, oxidation and dimerization, Cinzia Chiappe, Angelo Sanzone and Paul J. Dyson, Green Chem., 2011, 13, 1437-1441*
Keep up-to-date with the latest content in Green Chemistry by registering for our free table of contents alerts.
*Article free to access until the 13th February 2013.
Comments Off on Meet our Authors: Cinzia Chiappe


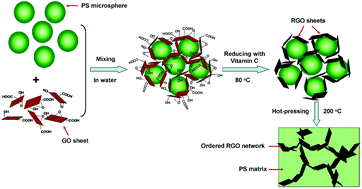
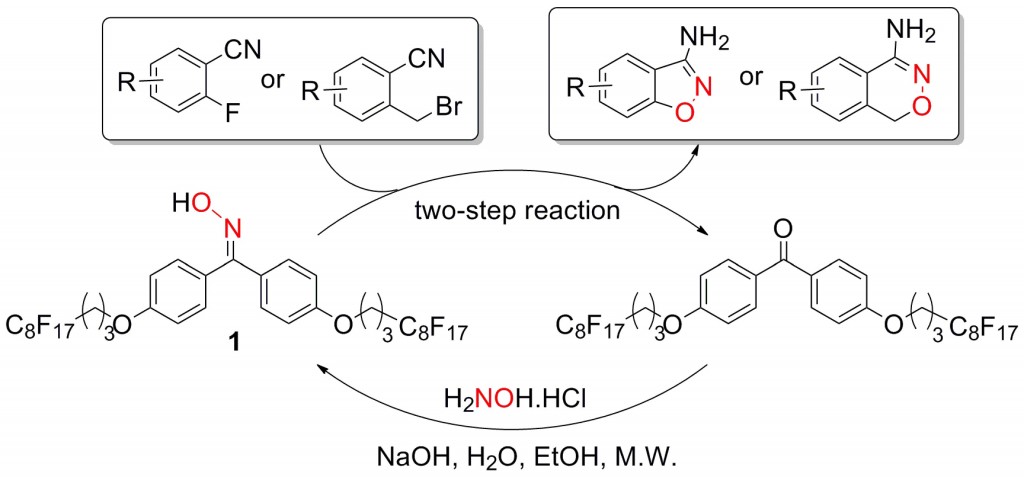
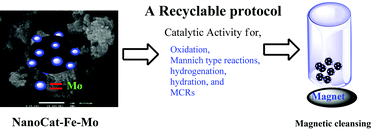
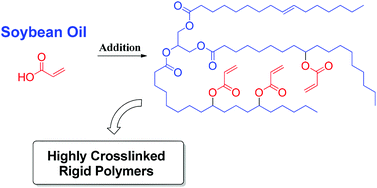









 This issue features work by
This issue features work by  The inside front cover features work by
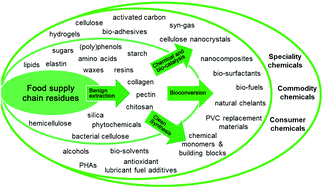
The inside front cover features work by 


 The latest addition of the “Green Solvents for Synthesis” conference took place in the picturesque Rhein Valley in Boppard, Germany from October 8-10, 2012. This biennial Dechema conference brings together world renowned chemists and engineers from both academia and industry to discuss their recent developments and future insights into the field of alternative solvents, solution phase chemistry, and processes. It is always held in a unique part of Germany, in which previous conferences have been held in the lower Rhein Valley (Bruchsal), Lake Constance (Friedrichshafen) and the Bavarian Alps (Berchtesgaden), and this time in the middle Rhein Valley. The beauty of Boppard and its surrounds (including the Loreley), a UNESCO Heritage site, was an excellent backdrop for the conference and emphasized the importance of sustainable development, an underlying theme of Green Chemistry.
The latest addition of the “Green Solvents for Synthesis” conference took place in the picturesque Rhein Valley in Boppard, Germany from October 8-10, 2012. This biennial Dechema conference brings together world renowned chemists and engineers from both academia and industry to discuss their recent developments and future insights into the field of alternative solvents, solution phase chemistry, and processes. It is always held in a unique part of Germany, in which previous conferences have been held in the lower Rhein Valley (Bruchsal), Lake Constance (Friedrichshafen) and the Bavarian Alps (Berchtesgaden), and this time in the middle Rhein Valley. The beauty of Boppard and its surrounds (including the Loreley), a UNESCO Heritage site, was an excellent backdrop for the conference and emphasized the importance of sustainable development, an underlying theme of Green Chemistry. As the year draws to a close, here is a list of the top 10 cited review articles in Green Chemistry in 2012 – all free to access until the
As the year draws to a close, here is a list of the top 10 cited review articles in Green Chemistry in 2012 – all free to access until the  The front cover of this month’s issue highlights the work of
The front cover of this month’s issue highlights the work of  The inside front cover features the work by
The inside front cover features the work by 