Experimental evidence for two-centre three-electron bond described as ‘a triumph of spectroscopy’
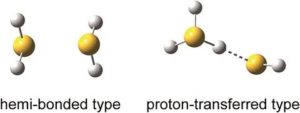
Researchers in Japan have observed the stable hemi-bonded structure of (H2S)n+ (n = 3–6). Using infra-red (IR) spectroscopy, the team has experimental evidence for this unusual, previously only theoretically predicted, structure.
The two-centre three-electron (2c–3e) bond, also known as a hemi bond, was first proposed by Linus Pauling in the 1930s. It is formed by the lone pair orbitals of a neutral molecule and its radical cation overlapping, causing the bonding sigma orbital to be doubly occupied and the antibonding sigma* orbital to be singly occupied.
Read the full story by Suzanne Howson on Chemistry World.