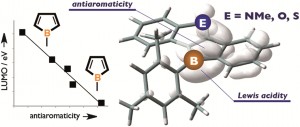
Aromaticity and antiaromaticity is one of the most fundamental concepts of chemistry but the nature of antiaromaticity remains elusive because of the lack of stable species.
A research team from Japan has provided an explanation for why heteroarene-fused boroles are higher in antiaromaticity and benzene-fused boroles are lower in antiaromaticity than the parent borole, providing a crucial design principle for more fascinating antiaromatic π-conjugated molecular systems.
Link to journal article
Heteroarene-fused Boroles: What Governs the Antiaromaticity and Lewis Acidity of the Borole Skeleton?
A Iida, A Sekioka and S Yamaguchi
Chem. Sci., 2012, DOI: 10.1039/c2sc20100c