Researchers from Wuhan University and the Lanzhou Institute of Chemical Physics have described a sulfur-promoted cleavage of aryl–iodo bonds by a Pd(ll) species.
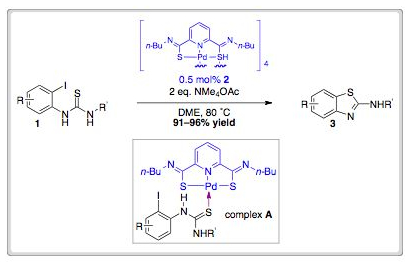
Aiwen Lei and co-workers showed that treatment of thioimido substrates (1) with a catalytic amount of a “pincer” Pd(ll) complex (2) at 80 ˚C could form thiazoles (3) in excellent yield.
In Nature, sulfur plays an important role in tuning the electronic properties of metals found in proteins. In analogy to the role of cysteine (a sulfur-containing amino acid), the incorporation of a donating sulfur ligand in the starting material (1) is thought to promote reaction of the Pd(ll) centre with the aryl-iodo bond. The reaction is thought to proceed via formation of complex A, whose structure has been confirmed by single crystal analysis.
Further investigations into the mechanism of this reaction are ongoing, but the researchers are hopeful that this work will enable new opportunities for the use of Pd(ll)–Pd(lV) chemistry, a powerful yet under-investigated catalysis mode.
Find out more by downloading Lei’s Edge article today.