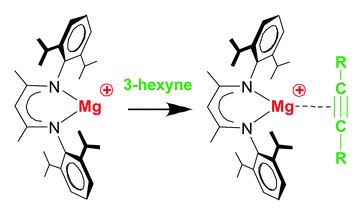
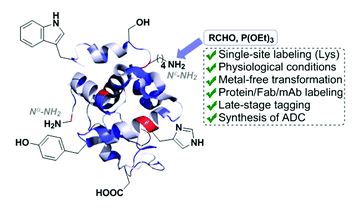
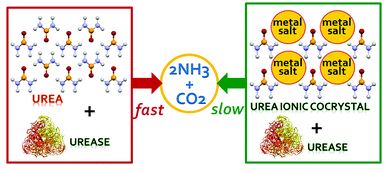
All of the referee-recommended articles below are free to access until 7th December 2018.
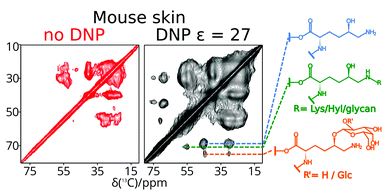
Essential but sparse collagen hydroxylysyl post-translational modifications detected by DNP NMR
Wing Ying Chow, Rui Li, Ieva Goldberga, David G. Reid, Rakesh Rajan, Jonathan Clark, Hartmut Oschkinat, Melinda J. Duer, Robert Hayward and Catherine M. Shanahan
Chem. Commun., 2018,54, 12570-12573
DOI: 10.1039/C8CC04960B, Communication
___________________________________________________________
Rapid synthesis of Co3O4 nanosheet arrays on Ni foam by in situ electrochemical oxidization of air-plasma engraved Co(OH)2 for efficient oxygen evolution
Wenling Gu, Liuyong Hu, Xiaoqing Zhu, Changshuai Shang, Jing Li and Erkang Wang
Chem. Commun., 2018, Advance Article
DOI: 10.1039/C8CC06399K, Communication
___________________________________________________________
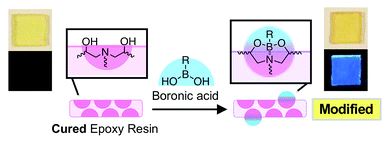
Modification of amine-cured epoxy resins by boronic acids based on their reactivity with intrinsic diethanolamine units
Yumiko Ito, Jumpei Kida, Daisuke Aoki and Hideyuki Otsuka
Chem. Commun., 2018, Advance Article
DOI: 10.1039/C8CC07412G, Communication
___________________________________________________________
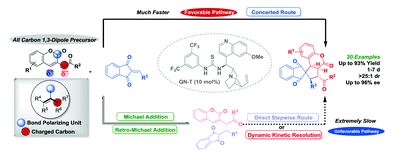
3-Homoacyl coumarin: an all carbon 1,3-dipole for enantioselective concerted (3+2) cycloaddition
Yi-Ru Chen, Madhusudhan Reddy Ganapuram, Kai-Hong Hsieh, Kai-Han Chen, Praneeth Karanam, Sandip Sambhaji Vagh, Yan-Cheng Liou and Wenwei Lin
Chem. Commun., 2018, Advance Article
DOI: 10.1039/C8CC07271J, Communication
___________________________________________________________
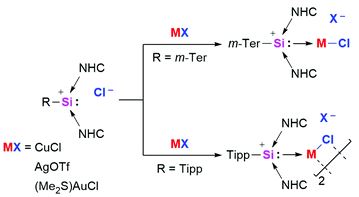
Coinage metal complexes of NHC-stabilized silyliumylidene ions
Philipp Frisch and Shigeyoshi Inoue
Chem. Commun., 2018, Advance Article
DOI: 10.1039/C8CC07754A, Communication
___________________________________________________________
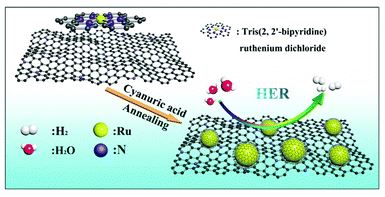
An ultrafine ruthenium nanocrystal with extremely high activity for the hydrogen evolution reaction in both acidic and alkaline media
Yutong Li, Fuqiang Chu, Yang Liu, Yong Kong, Yongxin Tao, Yongxin Li and Yong Qin
Chem. Commun., 2018, Advance Article
DOI: 10.1039/C8CC08276F, Communication












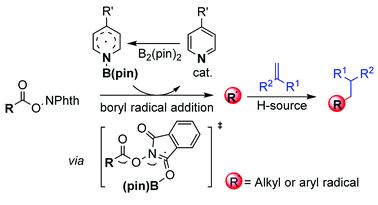














![[double bond, length as m-dash]](https://www.rsc.org/images/entities/h2_char_e001.gif) Ge
Ge![[double bond splayed right]](https://www.rsc.org/images/entities/h2_char_e00a.gif) double bond
double bond