Quantum dots are currently being developed for a variety of applications, including as sensors and cellular tags. Semiconductor quantum dots are attractive for their high fluorescence quantum yields but the toxicity of some of the metals involved, such as cadmium, pose a problem for biological applications.
Carbon quantum dots (CQDs) offer an alternative however when transferred into aqueous solution they possess low quantum yields. The problem is how do you harness the higher fluorescence of CQDs prepared in an organic solvent for biological applications?
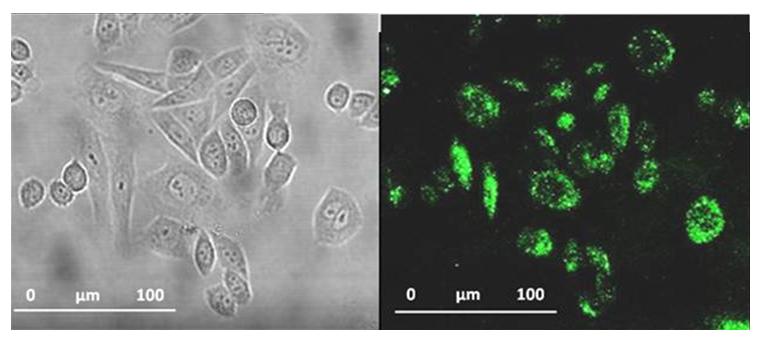
To answer this question John Callan and his team have employed an amphiphilic polymer to act as an overcoat and transfer agent for the CQDs. Surprisingly they found that the transfer actually improved the quantum yield rather than the normally expected repression when ligand exchange is used. These aqueous carbon quantum dots were taken up into cells and were found to be non-toxic.
The development of inexpensive and biocompatible quantum dots with an improved quantum yield holds great potential for a wide range of future biological applications.
To find out more, download the ChemComm article today (free to access for a limited period).












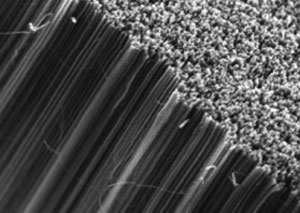 3D carbon nanotube forests are of particular interest in the electrochemical arenas of sensing and energy applications. Some researchers have suggested that it is necessary to use open-ended carbon nanotubes and carry out a pre-treatment or activation step to support fast electrochemistry, but is this always the case?
3D carbon nanotube forests are of particular interest in the electrochemical arenas of sensing and energy applications. Some researchers have suggested that it is necessary to use open-ended carbon nanotubes and carry out a pre-treatment or activation step to support fast electrochemistry, but is this always the case?