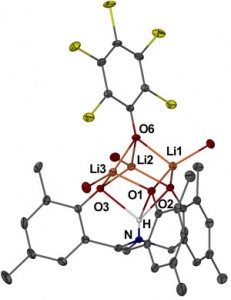
Now, Matthew Davidson and colleagues at the University of Bath in the UK have devised an organometallic synthetic strategy to make pseudocubane motifs of ammonium tris(phenol) ligands and lithium or sodium atoms, where one of the metals has been replaced by a hydrogen atom. The hydrogen forms the rarely reported trifurcated 4-centre hydrogen bond. Such an arrangement is not uncommon for the larger alkali metals, which can accommodate higher coordination numbers, but is not preferred by hydrogen.
Davidson says their interest lies in gaining a better understanding of how ligands like amine tris(phenolate) can be used to control reactive metal centres. A thorough comprehension of the coordination chemistry and the ability to draw conceptual similarities between the reactivity of hydrogen and metals as Lewis acids could help advance areas such as organocatalysis.
Read the full article in Chemistry World»
Read the original journal article in ChemComm:
Unprecedented participation of a four-coordinate hydrogen atom in the cubane core of lithium and sodium phenolates
David M. Cousins, Matthew G. Davidson and Daniel García-Vivó
Chem. Commun., 2014, Advance Article
DOI: 10.1039/C3CC47393G, Communication