The title of this post was taken from the website of Barry Trost, one of the world’s leading scientists and author of an astonishing 924 papers. Describing his work, he states:
One major activity in designing new reactions and reagents involves the development of “chemists’ enzymes” – non-peptidic transition metal based catalysts that can perform chemo-, regio-, diastereo-, and especially enantioselective reactions.
Chemists have, for a long time, sought to reproduce the incredible feats of nature. Natural biology has evolved over many years to achieve the efficiency and reactivity that most lab-based chemists could only dream of. Nature achieves this by employing incredibly sophisticated enzymes which are, sadly, almost impossible to replicate by a synthetic chemist due to their complexity. An alternative idea is to use these enzymes as inspiration for new catalysts and try to focus on the general reasons why they work rather than trying to create direct copies.
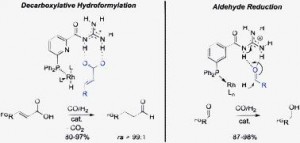
Dr Bernhard Breit, Lisa Diab and Urs Gellrich at Albert-Ludwigs-Univertat in Germany have shown in a HOT ChemComm article that a highly selective catalyst can be created when combining a metal catalyst with a directing ligand to control the reaction. In this Communication, they report excellent results using rhodium, the classic metal of choice for hydroformylation, and a functional group for recognition of the substrate. The net effect of these features combined is that the substrate is held in a specific way at the catalytic site. As a result, the reaction which follows can only occur in a specific way. This is similar to how enzymes control the chirality.
The concept behind this catalyst is one which could be applied to a great number of different reactions – no doubt we can look forward to reading about these in the near future.
Read this HOT ChemComm article today!
Tandem decarboxylative hydroformylation–hydrogenation reaction of α,β-unsaturated carboxylic acids toward aliphatic alcohols under mild conditions employing a supramolecular catalyst system
Lisa Diab, Urs Gellrich and Bernhard Breit
Chem. Commun., 2013, Advance Article
DOI: 10.1039/C3CC45547E, Communication
Ruaraidh McIntosh is a guest web-writer for ChemComm. His research interests include supramolecular chemistry and catalysis. When not working as a Research Fellow at Heriot-Watt University, Ruaraidh can usually be found in the kitchen where he has found a secondary application for his redoubtable skills in burning and profanity.