Have you ever wished for windows that clean themselves? One of the approaches to design such windows is to make them hydrophobic. On a hydrophobic surface, rain droplets will be more likely to roll over – taking the dirt particles with them. However, this process is not fully understood yet. Questions such as what happens when dirt and droplet collide, and what are the forces involved do not have a complete answer yet. Addressing such problems is of high importance both from a fundamental and applied point of view.
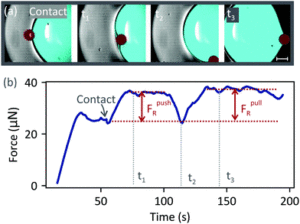
In this publication, the authors used an inverted confocal microscope to study the removal of dirt particles by a water drop deposited on a hydrophobic surface. The drop was held at a fixed position by a blade, while a dirt particle was moved at constant speed towards the drop. This setup allowed them to visualise the drop-particle collision, and measure the force acting on the drop during the collision, enabling the authors to assess the validity of existing models. The insights presented in the article contribute to a better understanding of the mechanisms involved, paving the way towards a future enhancement of self-cleaning surfaces.
Comments from the authors:
- When a drop collides with a particle on a surface, the drop successfully displaces the particle when the speed of the collision is low. Beyond a certain speed, the particle moves through the drop and leaves at its rear side.
- The force responsible for displacing the particle is the surface tension (or capillary force), which acts when the particle is at the drop’s interface. Particles experience a negligible viscous force when inside a water drop, because of the low viscosity of water. That is, the force due to the flow inside the drop is insufficient to displace the particle.
- The particle is displaced by the drop if the maximum capillary force that the drop can exert on the particle exceeds the resistive force that needs to be overcome to displace the particle over the surface.
- The maximum capillary force depends on the material properties of the liquid and of the particle, as well as how the particle moves (whether it rolls or slides).
- We developed a model which predicts that a rolling particle experiences a lower maximum capillary force than a sliding one.
- We observed that the particle rolled when it was pulled by the drop. There are two main contributions to the resistive force experienced by a rolling particle: one from the surface and the other from the drop. The first contribution is due to viscoelastic dissipation in the PDMS surface and due to intermolecular forces between the particle and the surface. The second contribution is due to contact angle hysteresis as the particle rolls at the drop-air interface.
- To maximise the chance of removing a particle from a surface using water drops, the resistive force experienced by the particle should be minimised. This can be achieved by lubricating the surface, or by coating it with a superhydrophobic material.
Citation to the paper: How a water drop removes a particle from a hydrophobic surface, Abhinav Naga, Anke Kaltbeitzel, William S. Y. Wong, Lukas Hauer, Hans-Jurgen Butt and Doris Vollmer. Soft Matter, 2021, 17, 1746. DOI: 10.1039/d0sm01925a.
To read the full article click here!
About the web writer
Dr Nacho Martin-Fabiani (@FabianiNacho) is a UKRI Future Leaders Fellow and Senior Lecturer in Materials Science at Loughborough University, UK.