Crystallization processes from a liquid are very present in our life, for example the freezing of water and formation of ice crystals. In such processes, building blocks suspended in a fluid arrange themselves to form a crystal. Espinosa et al. focus on the case where these building blocks are hard spherical particles.
In a real-life situation, the liquid will normally be confined within certain walls – e.g. inside a container or on top of a surface. Therefore, some of the particles will interact with the walls and might 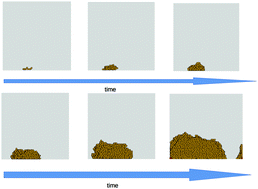 form a small crystal (or nucleus) at its surface – the so-called heterogeneous nucleation. This is more favourable than generating a crystal in the bulk of the liquid (homogeneous nucleation), as it reduces the surface area of the crystal and thus the energy required to form it. In this work, the authors study the competition between both nucleation mechanisms. They carry out molecular dynamics simulations to model heterogeneous nucleation processes and compare with existing homogeneous nucleation numerical data. This enables them to identify two regimes depending on the density of the system, and in each of them a different nucleation mechanism is prevalent. When the number of particles in the liquid is not too high (less than 53% in volume), heterogeneous nucleation prevails. But above that threshold homogeneous nucleation takes over.
form a small crystal (or nucleus) at its surface – the so-called heterogeneous nucleation. This is more favourable than generating a crystal in the bulk of the liquid (homogeneous nucleation), as it reduces the surface area of the crystal and thus the energy required to form it. In this work, the authors study the competition between both nucleation mechanisms. They carry out molecular dynamics simulations to model heterogeneous nucleation processes and compare with existing homogeneous nucleation numerical data. This enables them to identify two regimes depending on the density of the system, and in each of them a different nucleation mechanism is prevalent. When the number of particles in the liquid is not too high (less than 53% in volume), heterogeneous nucleation prevails. But above that threshold homogeneous nucleation takes over.
This study not only provides further insights into the competition between nucleation mechanisms; the expected prevalence of heterogeneous nucleation in experimental conditions could be part of the explanation as to why experimental and modelling results in the field have often discrepancies.
Comments from the authors:
• Above a certain density, a fluid composed hard spheres arranged in a disordered fashion is less stable than a crystal where the spheres are packed as cannon balls.
• The first step of the transformation into the crystal is the emergence of crystal embryos (or nuclei) in the fluid.
• Crystal nuclei may appear in the fluid bulk (homogeneous nucleation) or on the walls of the cell containing the fluid (heterogeneous nucleation).
• Whereas walls are always present in experiments, simulations effectively eliminate them with a trick called “periodic boundary conditions” by which a sphere leaving the simulation box on one side enters from the opposite side like ghosts in the Pacman game.
• The simultaneous appearance of homogeneous and heterogeneous nuclei makes it difficult to measure homogeneous nucleation rates in experiments.
• The balance between homogeneous and heterogeneous nucleation depends on the size and the shape of the container and on the fluid density.
• Heterogeneous nucleation prevails in fluids where particles occupy less than ~ 54 per cent of the space in cells typically used in experiments.
• The dominance of heterogeneous nucleation could explain long-standing discrepancies between experimental measurements and simulation estimates of the homogeneous nucleation rate.
• A strategy based on coating the cells with spheres arranged in a fluid fashion could potentially eliminate heterogeneous nucleation in experiments.
Citation to the paper: Heterogeneous versus homogeneous crystal nucleation of hard spheres, Soft Matter, 2019, 15, 9625-9631, DOI: 10.1039/C9SM01142K
To read the full article click here!
About the web writer
Dr Ignacio Martin-Fabiani (@FabianiNacho) is a Vice-Chancellor’s Research Fellow at the Department of Materials, Loughborough University, UK.