We are very pleased to introduce John McGrady, corresponding author, and his co-authors of the paper ‘The kinetics and mechanism of H2O2 decomposition at the U3O8 surface in bicarbonate solution‘. Their article has been very well received and handpicked by our reviewers and handling editors as one of our HOT articles. John told us more about the work that went into this article and what he hopes to achieve in the future. You can find out more about the authors and their article below and find more HOT articles in our online collection.
Meet the authors
Could you briefly explain the focus of your article to the non-specialist (in one or two sentences only) and why it is of current interest?
A key issue in the nuclear industry today is the disposal methodology of nuclear waste, with the direct disposal of spent nuclear fuel in deep geological facilities being an alternative option in Japan. This article focusses on predicting the potential release of radioactive material via dissolution of uranium upon contact of spent nuclear fuel with groundwater.
How big an impact could your results potentially have?
This study determined that the water chemistry of groundwater has a significant effect on the dissolution rate of highly oxidised uranium (U3O8) waste. As groundwater chemistry varies depending on deep geological facility location, the observed effects of groundwater chemistry on waste dissolution have significant implications for safety case formulation regarding predictions of radionuclide release into the environment. The results from this study also showed that the mechanism of dissolution is highly dependent on the form of oxidised uranium within the spent fuel, showing that the composition of uranium waste will have a large bearing on uranium release.
Could you explain the motivation behind this study?
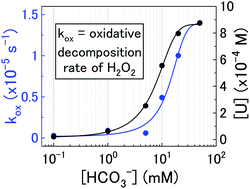
This study is part of a project being conducted in Japan regarding the development of the direct disposal method as an alternative option for the handling of spent nuclear fuel according to the present Japanese strategy. Currently, there is still a lack of knowledge regarding the mechanism of uranium dissolution from spent nuclear fuel and the effect of oxidants such as H2O2 on dissolution. Additionally, typical concentrations of bicarbonate in deep groundwater in Japan is over ten times higher than that of Western countries. Therefore, uranium dissolution with H2O2 as a function of bicarbonate has been investigated in this study.
In your opinion, what are the key design considerations for your study?
The key design consideration was how to simulate radiation effects to instigate the dissolution reactions. Radiation generated from decay of nuclides in nuclear waste will cause water radiolysis of groundwater and the formation of a number of oxidising species that affect dissolution. H2O2 was chosen as the main oxidant in this study, and as the concentration of H2O2 generated by nuclear waste induced radiolysis will depend on the lifetime of the waste, the concentration of H2O2 used was carefully considered.
Which part of the work towards this paper proved to be most challenging?
The most challenging part of this paper was combining the uranium dissolution data and H2O2 decomposition rates. However, we were able to produce a logical pathway for U3O8 dissolution by H2O2 in bicarbonate solution which described all of the observed experimental data.
What aspect of your work are you most excited about at the moment?
We have successfully investigated the dissolution of uranium oxide into solution with H2O2 and bicarbonate in anoxic conditions, and have determined the effect of groundwater chemistry and uranium oxide form on the dissolution rate of uranium. Dissolution is an interfacial process at the solid/liquid boundary, yet we do not currently know the exact nature of the surface oxide during the dissolution reaction. This provides an exciting challenge for future studies to characterize the surface in-situ during dissolution to further understand the observed dissolution behaviour of uranium.
What is the next step? What work is planned?
The next step in this research project is the in-situ characterisation of the surface of simulated waste during dissolution reactions using spectroscopic techniques. In real-time we plan to observe the evolution of the surface chemistry as dissolution proceeds, and use this information to provide a deeper understanding of the observed uranium release rates.
The kinetics and mechanism of H2O2 decomposition at the U3O8 surface in bicarbonate solution
John McGrady, Yuta Kumagai, Masayuki Watanabe, Akira Kirishima, Daisuke Akiyama, Akira Kitamura and Shingo Kimuro
RSC Adv., 2021,11, 28940-28948
DOI: 10.1039/D1RA05580A, Paper
 Submit to RSC Advances today! Check out our author guidelines for information on our article types or find out more about the advantages of publishing in a Royal Society of Chemistry journal.
Submit to RSC Advances today! Check out our author guidelines for information on our article types or find out more about the advantages of publishing in a Royal Society of Chemistry journal.
Keep up to date with our latest HOT articles, Reviews, Collections & more by following us on Twitter. You can also keep informed by signing up to our E-Alerts.