We are very pleased to introduce Ruel McKenzie, corresponding authors of the paper ‘Breaking the bottleneck: stilbene as a model compound for optimizing 6π e− photocyclization efficiency‘. His article has been very well received and handpicked by our reviewers and handling editors as one of our HOT articles. Ruel told us more about the work that went into this article and what he hopes to achieve in the future. You can find out more about the author and his article below and find more HOT articles in our online collection.
Meet the author
 Dr. Ruel McKenzie was born and raised in Kingston, Jamaica. He is an assistant professor in the School of Polymer Science & Polymer Engineering at The University of Akron. Prior to his current role, Dr. McKenzie was an NRC Postdoctoral Fellow at the Air Force Research Laboratory at Wright-Patterson Air Force Base and a postdoctoral researcher at the Foundation for Research and Technology-Hellas in the Institute of Electronic Structure and Laser. His degrees are in chemical engineering and he is a graduate of the NYU Tandon School of Engineering (B.Sc. and Ph.D.) and Columbia University (M. Sc.). Dr. McKenzie’s research activities are primarily in the field of chemical physics/physical chemistry of soft matter. His research is focused on making advances in the areas of soft matter dynamics, enabling complex structures and multifunctional materials.
Dr. Ruel McKenzie was born and raised in Kingston, Jamaica. He is an assistant professor in the School of Polymer Science & Polymer Engineering at The University of Akron. Prior to his current role, Dr. McKenzie was an NRC Postdoctoral Fellow at the Air Force Research Laboratory at Wright-Patterson Air Force Base and a postdoctoral researcher at the Foundation for Research and Technology-Hellas in the Institute of Electronic Structure and Laser. His degrees are in chemical engineering and he is a graduate of the NYU Tandon School of Engineering (B.Sc. and Ph.D.) and Columbia University (M. Sc.). Dr. McKenzie’s research activities are primarily in the field of chemical physics/physical chemistry of soft matter. His research is focused on making advances in the areas of soft matter dynamics, enabling complex structures and multifunctional materials.
Could you briefly explain the focus of your article to the non-specialist (in one or two sentences only) and why it is of current interest?
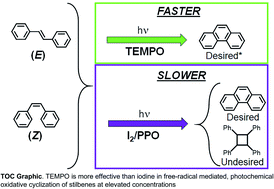
Stilbene was used as a model compound to mechanistically understand and overcome some factors that have limited 6π e− photocyclization reactions to dilute conditions – which essentially limited throughput. This is of interest because the synthesis of polycyclic aromatic compounds is typically conducted through such photochemical routes.
How big an impact could your results potentially have?
Polycyclic aromatic compounds have an array of emergent properties of interest to the materials science community. The results from this work will contribute to efforts to increase the production scale of polycyclic aromatic compounds that use similar photochemical routes. We demonstrated the utility of an alternative oxidizing agent to the convention which increased the throughput by over 10-fold and could be extended to high concentrations without the evolution of undesired products. We anticipate further work in screening other potential oxidizing agents that may be used for enhanced throughput. This work also highlighted the relevance of stereoconformation on the reaction dynamics and the impact of light source on the equilibrium conformation, especially as concentration increased.
Could you explain the motivation behind this study?
This study is part of a larger effort to understand and control the formation of complex architectures of polycyclic aromatic compounds and enabling synthesis of such compounds at high throughput using photochemical routes. The transformation of stilbene to phenanthrene represented the most basic molecular geometry that could be studied, and it afforded us the opportunity to monitor the photocyclization reaction in a straightforward manner.
In your opinion, what are the key design considerations for your study?
The key design considerations from this study were the impact of conformer and oxidizing agents on the reaction dynamics.
Which part of the work towards this paper proved to be most challenging?
Samples needed to be prepared in a dark room to maintain the integrity of the pure isomers and post-reaction removal of one of the oxidizing agents at high concentrations was a laborious process.
What aspect of your work are you most excited about at the moment?
We are currently excited about the revelation on the impact of the light source on the equilibrium stereoconformation, which is a design aspect of the reaction that appears to have been largely overlooked. The light source will drive the photoactive molecule to an equilibrium stereoconformation irrespective of the starting conformation. Understanding this relationship between physical aspects of the light source (such as wavelength and intensity) and equilibrium stereoconformation (or conformation pathway) will help to elucidate how complex structures are formed (or can be manipulated) during photochemical synthesis of polycyclic aromatic compounds.
What is the next step? What work is planned?
We are extending our current understanding of the mechanism of phenanthrene formation to study photocyclization in larger polycyclic aromatic molecules towards elucidating the mechanisms of forming complex geometries.
Breaking the bottleneck: stilbene as a model compound for optimizing 6π e− photocyclization efficiency
Joshua Seylar, Dmytro Stasiouk, Davide L. Simone, Vikas Varshney, James E. Heckler and Ruel McKenzie
RSC Adv., 2021,11, 6504-6508
DOI: 10.1039/D0RA10619D, Paper
 Submit to RSC Advances today! Check out our author guidelines for information on our article types or find out more about the advantages of publishing in a Royal Society of Chemistry journal.
Submit to RSC Advances today! Check out our author guidelines for information on our article types or find out more about the advantages of publishing in a Royal Society of Chemistry journal.
Keep up to date with our latest HOT articles, Reviews, Collections & more by following us on Twitter. You can also keep informed by signing up to our E-Alerts.