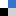Can hydrogenation reactions be performed in water? German scientists say: “Yes we can!”
Often, water is seen as the “green solvent” of choice as it is non-flammable and non-toxic. However, organic chemists usually face issues with the solubility of the reactants in water. In this RSC Advances article, Schwarze et al. demonstrate the power of the surfactants in a hydrogenation reaction of prochical C–C, double bonds in itaconate, e.g. dimethyl itaconate.
The selectivity were comparable to when the same reaction were performed in methanol. The reaction rates however were slightly slower. This was due to the lower hydrogen solubility in the micellar aqueous systems. But the beauty of the reaction is that no notable catalyst deactivation occurred and the latter was recyclable with a turn-over number of >1000 in the aqueous micellar conditions .
In this publication, the authors also developed a model to predict the performance of micellar reaction systems. The partition coefficient of the substrates between the micelles and the continuous aqueous phase can be predicted using the Conductor-like Screening Model for Real Solvents (COSMO-RS).
If you want to find out more about this work, please read the full paper here*.
Rhodium catalyzed hydrogenation reactions in aqueous micellar systems as green solvents
M. Schwarze, J.S. Milano-Brusco, V. Strempel, T. Hamerla, S. Wille, C. Fischer, W. Baumann, W. Arlt and R. Schomäcker
RSC Adv., 2011, DOI: 10.1039/C1RA00397F, Advance Article
*Individuals can access the content by signing up for an RSC Publishing Personal Account. Existing institutional RSC journal subscribers, with registered IP, have automatic access. Other institutions can register for free access.